Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy
- PMID: 27698936
- PMCID: PMC5039339
- DOI: 10.7150/thno.15102
Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy
Abstract
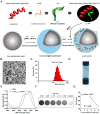
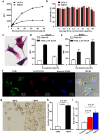
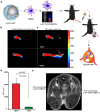
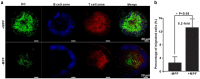
Dendritic cell (DC) migration to the lymph node is a key component of DC-based immunotherapy. However, the DC homing rate to the lymphoid tissues is poor, thus hindering the DC-mediated activation of antigen-specific T cells. Here, we developed a system using fluorescent magnetic nanoparticles (α-AP-fmNPs; loaded with antigen peptide, iron oxide nanoparticles, and indocyanine green) in combination with magnetic pull force (MPF) to successfully manipulate DC migration in vitro and in vivo. α-AP-fmNPs endowed DCs with MPF-responsiveness, antigen presentation, and simultaneous optical and magnetic resonance imaging detectability. We showed for the first time that α-AP-fmNP-loaded DCs were sensitive to MPF, and their migration efficiency could be dramatically improved both in vitro and in vivo through MPF treatment. Due to the enhanced migration of DCs, MPF treatment significantly augmented antitumor efficacy of the nanoparticle-loaded DCs. Therefore, we have developed a biocompatible approach with which to improve the homing efficiency of DCs and subsequent anti-tumor efficacy, and track their migration by multi-modality imaging, with great potential applications for DC-based cancer immunotherapy.
Keywords: DC migration tracking; DC vaccine; antigen delivery; immunotherapy; magnetic targeting..
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Sabado RL, Bhardwaj N. Cancer immunotherapy: dendritic-cell vaccines on the move. Nature. 2015;519:300–1. - PubMed
-
- Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. - PubMed
-
- van den Ancker W, van Luijn MM, Westers TM, Bontkes HJ, Ruben JM, de Gruijl TD. et al. Recent advances in antigen-loaded dendritic cell-based strategies for treatment of minimal residual disease in acute myeloid leukemia. Immunotherapy. 2010;2:69–83. - PubMed
-
- Zhou FF, Nordquist RE, Chen WR. Photonics immunotherapy — A novel strategy for cancer treatment. J Innov Opt Health Sci. 2016;9:1630001.
-
- Kastenmuller W, Kastenmuller K, Kurts C, Seder RA. Dendritic cell-targeted vaccines-hope or hype? Nat Rev Immunol. 2014;14:705–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

