Crucial Role of miR-433 in Regulating Cardiac Fibrosis
- PMID: 27698941
- PMCID: PMC5039681
- DOI: 10.7150/thno.15007
Crucial Role of miR-433 in Regulating Cardiac Fibrosis
Erratum in
-
Erratum: Crucial Role of miR-433 in Regulating Cardiac Fibrosis: Erratum.Theranostics. 2021 Jun 8;11(15):7618-7619. doi: 10.7150/thno.63330. eCollection 2021. Theranostics. 2021. PMID: 34158871 Free PMC article.
Abstract
Dysregulation of microRNAs has been implicated in many cardiovascular diseases including fibrosis. Here we report that miR-433 was consistently elevated in three models of heart disease with prominent cardiac fibrosis, and was enriched in fibroblasts compared to cardiomyocytes. Forced expression of miR-433 in neonatal rat cardiac fibroblasts increased proliferation and their differentiation into myofibroblasts as determined by EdU incorporation, α-SMA staining, and expression levels of fibrosis-associated genes. Conversely, inhibition of miR-433 exhibited opposite results. AZIN1 and JNK1 were identified as two target genes of miR-433. Decreased level of AZIN1 activated TGF-β1 while down-regulation of JNK1 resulted in activation of ERK and p38 kinase leading to Smad3 activation and ultimately cardiac fibrosis. Importantly, systemic neutralization of miR-433 or adeno-associated virus 9 (AAV9)-mediated cardiac transfer of a miR-433 sponge attenuated cardiac fibrosis and ventricular dysfunction following myocardial infarction. Thus, our work suggests that miR-433 is a potential target for amelioration of cardiac fibrosis.
Keywords: AZIN1; JNK1.; cardiac fibrosis; miR-433.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



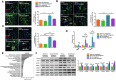
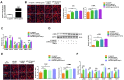

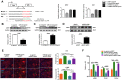
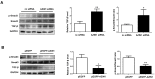



Comment in
-
Modulating microRNAs as Novel Therapeutic Targets in Cardiac Fibrosis.Theranostics. 2017 Jun 1;7(8):2287-2288. doi: 10.7150/thno.19286. eCollection 2017. Theranostics. 2017. PMID: 28740551 Free PMC article.
References
-
- Thum T. Noncoding rnas and myocardial fibrosis. Nat Rev Cardioy. 2014;11:655–63. - PubMed
-
- Leask A. Getting to the heart of the matter: New insights into cardiac fibrosis. Cir Res. 2015;116:1269–76. - PubMed
-
- Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev. 2014;19:173–85. - PubMed
-
- Thum T, Lorenzen JM. Cardiac fibrosis revisited by microrna therapeutics. Circulation. 2012;126:800–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

