Gelatin-based Hydrogel Degradation and Tissue Interaction in vivo: Insights from Multimodal Preclinical Imaging in Immunocompetent Nude Mice
- PMID: 27698944
- PMCID: PMC5039684
- DOI: 10.7150/thno.16614
Gelatin-based Hydrogel Degradation and Tissue Interaction in vivo: Insights from Multimodal Preclinical Imaging in Immunocompetent Nude Mice
Abstract
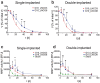
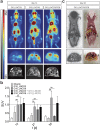
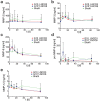
Hydrogels based on gelatin have evolved as promising multifunctional biomaterials. Gelatin is crosslinked with lysine diisocyanate ethyl ester (LDI) and the molar ratio of gelatin and LDI in the starting material mixture determines elastic properties of the resulting hydrogel. In order to investigate the clinical potential of these biopolymers, hydrogels with different ratios of gelatin and diisocyanate (3-fold (G10_LNCO3) and 8-fold (G10_LNCO8) molar excess of isocyanate groups) were subcutaneously implanted in mice (uni- or bilateral implantation). Degradation and biomaterial-tissue-interaction were investigated in vivo (MRI, optical imaging, PET) and ex vivo (autoradiography, histology, serum analysis). Multimodal imaging revealed that the number of covalent net points correlates well with degradation time, which allows for targeted modification of hydrogels based on properties of the tissue to be replaced. Importantly, the degradation time was also dependent on the number of implants per animal. Despite local mechanisms of tissue remodeling no adverse tissue responses could be observed neither locally nor systemically. Finally, this preclinical investigation in immunocompetent mice clearly demonstrated a complete restoration of the original healthy tissue.
Keywords: Autoradiography ex vivo; Biomaterials; Computed tomography; Magnetic resonance imaging; Optical imaging; Positron emission tomography..
Conflict of interest statement
Competing Interest: The authors declare no competing financial interests.
Figures



Similar articles
-
Interplay between stiffness and degradation of architectured gelatin hydrogels leads to differential modulation of chondrogenesis in vitro and in vivo.Acta Biomater. 2018 Mar 15;69:83-94. doi: 10.1016/j.actbio.2018.01.025. Epub 2018 Jan 31. Acta Biomater. 2018. PMID: 29378326
-
Protein composition alters in vivo resorption of PEG-based hydrogels as monitored by contrast-enhanced MRI.Biomaterials. 2015 Feb;42:1-10. doi: 10.1016/j.biomaterials.2014.11.015. Epub 2014 Dec 9. Biomaterials. 2015. PMID: 25542788
-
Characterization of Tissue Transglutaminase as a Potential Biomarker for Tissue Response toward Biomaterials.ACS Biomater Sci Eng. 2019 Nov 11;5(11):5979-5989. doi: 10.1021/acsbiomaterials.9b01299. Epub 2019 Oct 18. ACS Biomater Sci Eng. 2019. PMID: 33405720
-
Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties.Biomaterials. 2014 Dec;35(37):9755-9766. doi: 10.1016/j.biomaterials.2014.08.023. Epub 2014 Sep 5. Biomaterials. 2014. PMID: 25199786
-
Label-free imaging of gelatin-containing hydrogel scaffolds.Biomaterials. 2015 Feb;42:144-50. doi: 10.1016/j.biomaterials.2014.11.050. Epub 2014 Dec 16. Biomaterials. 2015. PMID: 25542802 Free PMC article.
Cited by
-
Convergent synthesis of diversified reversible network leads to liquid metal-containing conductive hydrogel adhesives.Nat Commun. 2021 Apr 23;12(1):2407. doi: 10.1038/s41467-021-22675-2. Nat Commun. 2021. PMID: 33893308 Free PMC article.
-
Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization.Theranostics. 2018 Oct 6;8(18):5159-5177. doi: 10.7150/thno.27760. eCollection 2018. Theranostics. 2018. PMID: 30429892 Free PMC article.
-
Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials.Polymers (Basel). 2023 Jan 24;15(3):603. doi: 10.3390/polym15030603. Polymers (Basel). 2023. PMID: 36771903 Free PMC article.
-
Induction of 4D spatiotemporal geometric transformations in high cell density tissues via shape changing hydrogels.Adv Funct Mater. 2021 Jun 9;31(24):2010104. doi: 10.1002/adfm.202010104. Epub 2021 Feb 24. Adv Funct Mater. 2021. PMID: 34335134 Free PMC article.
-
Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation.J Nanobiotechnology. 2024 Oct 24;22(1):653. doi: 10.1186/s12951-024-02916-4. J Nanobiotechnology. 2024. PMID: 39443923 Free PMC article.
References
-
- Rosiak JM, Yoshii F. Hydrogels and their medical applications. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms. 1999;151:56–64.
-
- Neffe AT, Pierce BF, Tronci G. et al. One Step Creation of Multifunctional 3D Architectured Hydrogels Inducing Bone Regeneration. Adv Mater. 2015;27:1738–1744. - PubMed
-
- Sartori S, Chiono V, Tonda-Turo C. et al. Biomimetic polyurethanes in nano and regenerative medicine. J Mater Chem B. 2014;2:5128–5144. - PubMed
-
- Patino MG, Neiders ME, Andreana S. et al. Collagen as an implantable material in medicine and dentistry. J Oral Implantol. 2002;28:220–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

