A Comparison between Hybrid and Concomitant Regimens for Helicobacter Pylori Eradication: A Randomized Clinical Trial
- PMID: 27698972
- PMCID: PMC5045675
- DOI: 10.15171/mejdd.2016.24
A Comparison between Hybrid and Concomitant Regimens for Helicobacter Pylori Eradication: A Randomized Clinical Trial
Abstract
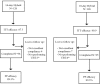
BACKGROUND Helicobacter pylori (H. pylori) is one of the most common bacterial infections worldwide. We designed a study to compare the efficacy of 14-day hybrid regimen with 10-day concomitant therapy for H. pylori eradication in Iran. METHODS 252 patients with naïve H. pylori infection were randomly divided to receive either hybrid regimen (pantoprazole 40 mg, and amoxicillin 1 gr twice daily for 14 days, accompanied by clarithromycin 500 mg, and metronidazole 500 mg, twice daily just during the last 7 days) or concomitant regimen (pantoprazole 40 mg, amoxicillin 1 gr, clarithromycin 500 mg, and metronidazole 500 mg, all twice daily for 10 days). 8 weeks after therapy, 14C- urease breath test was performed to confirm eradication. RESULTS According to intention to treat analysis, the eradication rates were 87.3% (95% CI: 81.4-93.1) and 80.9% (95% CI: 74-87.8) in hybrid and concomitant groups, respectively (p=0.38). Per-protocol eradication rates were 89.3% (95% CI: 83.8-94.7) and 83.1% (95% CI: 76.3-89.8), respectively (p=0.19). The rates of severe side effects were not statistically different between the two groups (4% vs. 8.7%). CONCLUSION 14-day hybrid therapy can be considered as a nearly acceptable regimen with few severe side effects in Iran. However, it seems that the efficacy of this therapy is decreasing as the resistance rates to antibiotics are increasing. We suggest further studies to assess the efficacy of a more prolonged concomitant therapy for H. pylori eradication in Iran.
Similar articles
-
Comparison Between 10- and 14-Day Hybrid Regimens for Helicobacter pylori Eradication: A Randomized Clinical Trial.Helicobacter. 2015 Aug;20(4):299-304. doi: 10.1111/hel.12202. Epub 2015 Mar 4. Helicobacter. 2015. PMID: 25752357 Clinical Trial.
-
A Comparison between 10-day and 12-day Concomitant Regimens for Helicobacter Pylori Eradication: A Randomized Clinical Trial.Middle East J Dig Dis. 2020 Apr;12(2):106-110. doi: 10.34172/mejdd.2020.169. Middle East J Dig Dis. 2020. PMID: 32626563 Free PMC article.
-
Non-inferiority of reverse hybrid regimen versus standard concomitant regimen for H. pylori eradication in a randomized-controlled trial.Caspian J Intern Med. 2023 Fall;14(4):687-693. doi: 10.22088/cjim.14.4.687. Caspian J Intern Med. 2023. PMID: 38024170 Free PMC article.
-
Reverse sequential therapy achieves a similar eradication rate as standard sequential therapy for Helicobacter pylori eradication: a randomized controlled trial.Helicobacter. 2015 Feb;20(1):71-7. doi: 10.1111/hel.12176. Epub 2014 Dec 11. Helicobacter. 2015. PMID: 25495272 Clinical Trial.
-
Hybrid Therapy Regimen for Helicobacter Pylori Eradication.Chin Med J (Engl). 2016 Apr 20;129(8):992-9. doi: 10.4103/0366-6999.179803. Chin Med J (Engl). 2016. PMID: 27064046 Free PMC article. Review.
Cited by
-
Efficacy of 14-day concomitant quadruple therapy and 14-day high-dose dual therapy on H. pylori eradication.Gastroenterol Hepatol Bed Bench. 2022 Spring;15(2):172-178. Gastroenterol Hepatol Bed Bench. 2022. PMID: 35845300 Free PMC article.
-
Effects of 14-days bismuth- and tetracycline-containing quadruple therapy with concomitant regimen for the first line Helicobacterpylori eradication.Caspian J Intern Med. 2023 Fall;14(4):676-680. doi: 10.22088/cjim.14.4.676. Caspian J Intern Med. 2023. PMID: 38024162 Free PMC article.
-
Equivalence Trial of the Non-Bismuth 10-Day Concomitant and 14-Day Hybrid Therapies for Helicobacter pylori Eradication in High Clarithromycin Resistance Areas.Antibiotics (Basel). 2024 Mar 20;13(3):280. doi: 10.3390/antibiotics13030280. Antibiotics (Basel). 2024. PMID: 38534715 Free PMC article.
-
Fourth-generation quinolones in the treatment of Helicobacter pylori infection: A meta-analysis.World J Gastroenterol. 2018 Aug 7;24(29):3302-3312. doi: 10.3748/wjg.v24.i29.3302. World J Gastroenterol. 2018. PMID: 30090010 Free PMC article.
-
Treatment of Helicobacter pylori Is Not Associated With Future Clostridium difficile Infection.Am J Gastroenterol. 2020 May;115(5):716-722. doi: 10.14309/ajg.0000000000000626. Am J Gastroenterol. 2020. PMID: 32356953 Free PMC article.
References
-
- Becker SI, Smalligan RD, Frame JD, Kleanthous H, Tibbitts TJ, Monath TP. et al. Risk of Helicobacter pylori infection among long-term residents in developing countries. Am J Trop Med Hyg. 1999;60:267–70. - PubMed
-
- Tokunaga K, Tanaka A, Sugano H, Takahashi S. [The present status and problems of Helicobacter pylori first-line eradication therapy] Nihon Rinsho. 2009;67:2291–6. - PubMed
-
- Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139–45. doi: 10.1111/j.1523-5378.2011.00828.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources