Guanosine: a Neuromodulator with Therapeutic Potential in Brain Disorders
- PMID: 27699087
- PMCID: PMC5036959
- DOI: 10.14336/AD.2016.0208
Guanosine: a Neuromodulator with Therapeutic Potential in Brain Disorders
Abstract
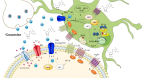
Guanosine is a purine nucleoside with important functions in cell metabolism and a protective role in response to degenerative diseases or injury. The past decade has seen major advances in identifying the modulatory role of extracellular action of guanosine in the central nervous system (CNS). Evidence from rodent and cell models show a number of neurotrophic and neuroprotective effects of guanosine preventing deleterious consequences of seizures, spinal cord injury, pain, mood disorders and aging-related diseases, such as ischemia, Parkinson's and Alzheimer's diseases. The present review describes the findings of in vivo and in vitro studies and offers an update of guanosine effects in the CNS. We address the protein targets for guanosine action and its interaction with glutamatergic and adenosinergic systems and with calcium-activated potassium channels. We also discuss the intracellular mechanisms modulated by guanosine preventing oxidative damage, mitochondrial dysfunction, inflammatory burden and modulation of glutamate transport. New and exciting avenues for future investigation into the protective effects of guanosine include characterization of a selective guanosine receptor. A better understanding of the neuromodulatory action of guanosine will allow the development of therapeutic approach to brain diseases.
Keywords: adenosine; glutamate; guanosine; neuromodulator; neuroprotection; neurotrophic effects; purines.
Figures


References
-
- Lippman F (1941). Metabolic generation and utilization of phosphate bond energy. Adv Enzymol, 1: 99-162
-
- Drury AN (1936). The physiological activity of nucleic acid and its derivatives. Physiol Rev, 16: 292-325
-
- Burnstock G (1972). Purinergic nerves. Pharmacological reviews, 24: 509-581 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
