Chemotherapeutic drug-specific alteration of microvascular blood flow in murine breast cancer as measured by diffuse correlation spectroscopy
- PMID: 27699124
- PMCID: PMC5030036
- DOI: 10.1364/BOE.7.003610
Chemotherapeutic drug-specific alteration of microvascular blood flow in murine breast cancer as measured by diffuse correlation spectroscopy
Abstract
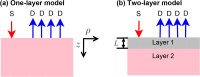
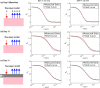
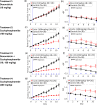
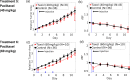
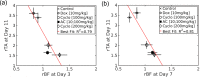
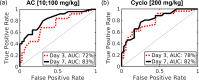
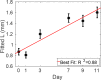
The non-invasive, in vivo measurement of microvascular blood flow has the potential to enhance breast cancer therapy monitoring. Here, longitudinal blood flow of 4T1 murine breast cancer (N=125) under chemotherapy was quantified with diffuse correlation spectroscopy based on layer models. Six different treatment regimens involving doxorubicin, cyclophosphamide, and paclitaxel at clinically relevant doses were investigated. Treatments with cyclophosphamide increased blood flow as early as 3 days after administration, whereas paclitaxel induced a transient blood flow decrease at 1 day after administration. Early blood flow changes correlated strongly with the treatment outcome and distinguished treated from untreated mice individually for effective treatments.
Keywords: (170.3660) Light propagation in tissues; (170.6480) Spectroscopy, speckle; (290.4210) Multiple scattering.
Figures


References
-
- Rastogi P., Anderson S. J., Bear H. D., Geyer C. E., Kahlenberg M. S., Robidoux A., Margolese R. G., Hoehn J. L., Vogel V. G., Dakhil S. R., Tamkus D., King K. M., Pajon E. R., Wright M. J., Robert J., Paik S., Mamounas E. P., Wolmark N., “Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27,” J. Clin. Onc. 26, 778–785 (2008).10.1200/JCO.2007.15.0235 - DOI - PubMed
-
- Caudle A. S., Gonzalez-Angulo A. M., Hunt K. K., Liu P., Pusztai L., Symmans W. F., Kuerer H. M., Mittendorf E. A., Hortobagyi G. N., Meric-Bernstam F., “Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer,” J. Clin. Onc. 28, 1821–1828 (2010).10.1200/JCO.2009.25.3286 - DOI - PMC - PubMed
-
- Yeh E., Slanetz P., Kopans D. B., Rafferty E., Georgian-Smith D., Moy L., Halpern E., Moore R., Kuter I., Taghian A., “Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer,” Am. J. Roentgenol. 184, 868–877 (2005).10.2214/ajr.184.3.01840868 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
