Preclinical photoacoustic models: application for ultrasensitive single cell malaria diagnosis in large vein and artery
- PMID: 27699126
- PMCID: PMC5030038
- DOI: 10.1364/BOE.7.003643
Preclinical photoacoustic models: application for ultrasensitive single cell malaria diagnosis in large vein and artery
Abstract
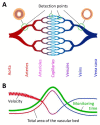
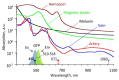
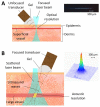
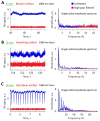
In vivo photoacoustic flow cytometry (PAFC) has demonstrated potential for early diagnosis of deadly diseases through detection of rare circulating tumor cells, pathogens, and clots in nearly the entire blood volume. Before clinical application, this promising diagnostic platform requires verification and optimization using adequate preclinical models. We show here that this can be addressed by examination of large mouse blood vessels which are similar in size, depth and flow velocity to human vessels used in PAFC. Using this model, we verified the capability of PAFC for ultrasensitive, noninvasive, label-free, rapid malaria diagnosis. The time-resolved detection of delayed PA signals from deep vessels provided complete elimination of background from strongly pigmented skin. We discovered that PAFC's sensitivity is higher during examination of infected cells in arteries compared to veins at similar flow rate. Our advanced PAFC platform integrating a 1060 nm laser with tunable pulse rate and width, a wearable probe with a focused transducer, and linear and nonlinear nanobubble-amplified signal processing demonstrated detection of parasitemia at the unprecedented level of 0.00000001% within 20 seconds and the potential to further improve the sensitivity 100-fold in humans, that is approximately 106 times better than in existing malaria tests.
Keywords: (110.5125) Photoacoustics; (330.6130) Spatial resolution.
Figures

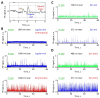
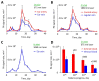

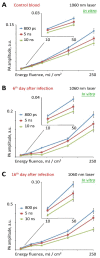
References
-
- Galanzha E. I., Shashkov E. V., Spring P. M., Suen J. Y., Zharov V. P., “In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser,” Cancer Res. 69(20), 7926–7934 (2009).10.1158/0008-5472.CAN-08-4900 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
