Morbility, clinical data and proteomic analysis of IUGR and AGA newborns at different gestational ages
- PMID: 27699198
- PMCID: PMC5037120
- DOI: 10.1016/j.dib.2016.09.024
Morbility, clinical data and proteomic analysis of IUGR and AGA newborns at different gestational ages
Abstract
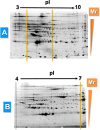
The data are related to the proteomic analysis of 43 newborns with intrauterine growth retardation (IUGR) and 45 newborns with appropriate weight for gestational age (AGA) carried out by separation via 2DE and analyzed by MS-TOF/TOF. All newborns were separated into three gestational age groups, "Very Preterm" 29-32 weeks, "Moderate Preterm" 33-36 weeks, and, "Term" ≥37weeks. From each newborn, blood was drawn three times from birth to 1 month life. High-abundant serum proteins were depleted, and the minority ones were separated by 2DE and analyzed for significant expression differences. The data reflect analytic and clinic variables analyzed globally and categorized by gestational age in relation to IUGR and the optimization of conditions for 2-DE separation. The data from this study are related to the research article entitled "Alterations of Protein Expression in Serum of Infants with Intrauterine Growth Restriction and Different Gestational Ages" (M.D. Ruis-González, M.D. Cañete, J.L. Gómez-Chaparro, N. Abril, R. Cañete, J. López-Barea, 2015) [1]. The present dataset of serum IUGR newborn proteome can be used as a reference for any study involving intrauterine growth restriction during the first month of life.
Keywords: Human newborn; Intrauterine growth restriction; Proteomics; Serum proteins; Venous blood.
Figures
References
-
- Ruis-González M.D., Cañete M.D., Gómez-Chaparro J.L., Abril N., Cañete R., López-Barea J. Alterations of protein expression in serum of infants with intrauterine growth restriction and different gestational ages. J. Proteom. 2015;24(119):169–182. - PubMed
-
- Carrascosa A., Ferrández A., Yeste D., García-Dihinx J., Romo A., Copil A. Vol. 68. Barc; 2008. Estudio transversal español de crecimiento 2008. Parte I: valores de peso y longitud en recién nacidos de 26-42 semanas de edad gestacional; pp. 544–551. (An. Pediatr.). - PubMed
-
- Spanish Society of Gynaecology and Obstetrics (SEGO) (homepage on the internet). Madrid: Crecimiento intrauterino restringido. SEGO assistance protocols in 〈http://www.sego.es〉
-
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL 〈https://www.R-project.org/〉.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous