Quantum coherence spectroscopy to measure dietary fat retention in the liver
- PMID: 27699229
- PMCID: PMC5035097
- DOI: 10.1172/jci.insight.84671
Quantum coherence spectroscopy to measure dietary fat retention in the liver
Abstract
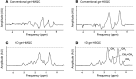
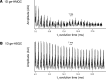
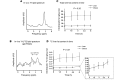
The prevalence of fatty liver reaches alarming proportions. Fatty liver increases the risk for insulin resistance, cardiovascular disease, and nonalcoholic steatohepatitis (NASH). Although extensively studied in a preclinical setting, the lack of noninvasive methodologies hampers our understanding of which pathways promote hepatic fat accumulation in humans. Dietary fat retention is one of the pathways that may lead to fatty liver. The low (1.1%) natural abundance (NA) of carbon-13 (13C) allows use of 13C-enriched lipids for in vivo MR studies. Successful implementation of such methodology, however, is challenging due to low sensitivity of 13C-magnetic resonance spectroscopy (13C-MRS). Here, we investigated the use of 1-dimensional gradient enhanced heteronuclear single quantum coherence (ge-HSQC) spectroscopy for the in vivo detection of hepatic 1H-[13C]-lipid signals after a single high-fat meal with 13C-labeled fatty acids in 5 lean and 6 obese subjects. Postprandial retention of orally administered 13C-labeled fatty acids was significant (P < 0.01). Approximately 1.5% of the tracer was retained in the liver after 6 hours, and retention was similar in both groups (P = 0.92). Thus, a substantial part of the liver fat can originate directly from storage of meal-derived fat. The ge-HSQC can be used to noninvasively reveal the contribution of dietary fat to the development of hepatic steatosis over time.
Figures


References
-
- Szczepaniak LS, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 Pt 1):E977–E989. - PubMed
-
- Seppälä-Lindroos A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87(7):3023–3028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

