The antifibrotic drug pirfenidone promotes pulmonary cavitation and drug resistance in a mouse model of chronic tuberculosis
- PMID: 27699232
- PMCID: PMC5033951
- DOI: 10.1172/jci.insight.86017
The antifibrotic drug pirfenidone promotes pulmonary cavitation and drug resistance in a mouse model of chronic tuberculosis
Abstract
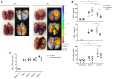
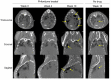
Pirfenidone is a recently approved antifibrotic drug for the treatment of idiopathic pulmonary fibrosis (IPF). Because tuberculosis (TB) is characterized by granulomatous inflammation in conjunction with parenchymal destruction and replacement fibrosis, we sought to determine whether the addition of pirfenidone as an adjunctive, host-directed therapy provides a beneficial effect during antimicrobial treatment of TB. We hypothesized that pirfenidone's antiinflammatory and antifibrotic properties would reduce inflammatory lung damage and increase antimicrobial drug penetration in granulomas to accelerate treatment response. The effectiveness of adjunctive pirfenidone during TB drug therapy was evaluated using a murine model of chronic TB. Mice treated with standard therapy 2HRZ/4HR (H, isoniazid; R, rifampin; and Z, pyrazinamide) were compared with 2 alternative regimens containing pirfenidone (Pf) (2HRZPf/4HRPf and 2HRZPf/4HR). Contrary to our hypothesis, adjunctive pirfenidone use leads to reduced bacterial clearance and increased relapse rates. This treatment failure is closely associated with the emergence of isoniazid monoresistant bacilli, increased cavitation, and significant lung pathology. While antifibrotic agents may eventually be used as part of adjunctive host-directed therapy of TB, this study clearly demonstrates that caution must be exercised. Moreover, as pirfenidone becomes more widely used in clinical practice, increased patient monitoring would be required in endemic TB settings.
Figures




References
-
- Editorial Fibrosis in tuberculosis. Journal of the American Medical Association. 1927;89 doi: 10.1001/jama.1927.02690200046016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

