Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications
- PMID: 27699244
- PMCID: PMC5033761
- DOI: 10.1172/jci.insight.86976
Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications
Abstract
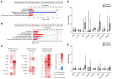
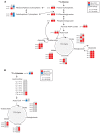
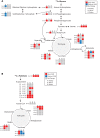
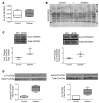
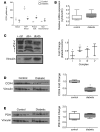
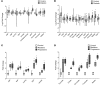
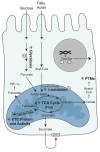
Diabetes is associated with altered cellular metabolism, but how altered metabolism contributes to the development of diabetic complications is unknown. We used the BKS db/db diabetic mouse model to investigate changes in carbohydrate and lipid metabolism in kidney cortex, peripheral nerve, and retina. A systems approach using transcriptomics, metabolomics, and metabolic flux analysis identified tissue-specific differences, with increased glucose and fatty acid metabolism in the kidney, a moderate increase in the retina, and a decrease in the nerve. In the kidney, increased metabolism was associated with enhanced protein acetylation and mitochondrial dysfunction. To confirm these findings in human disease, we analyzed diabetic kidney transcriptomic data and urinary metabolites from a cohort of Southwestern American Indians. The urinary findings were replicated in 2 independent patient cohorts, the Finnish Diabetic Nephropathy and the Family Investigation of Nephropathy and Diabetes studies. Increased concentrations of TCA cycle metabolites in urine, but not in plasma, predicted progression of diabetic kidney disease, and there was an enrichment of pathways involved in glycolysis and fatty acid and amino acid metabolism. Our findings highlight tissue-specific changes in metabolism in complication-prone tissues in diabetes and suggest that urinary TCA cycle intermediates are potential prognostic biomarkers of diabetic kidney disease progression.
Figures


References
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed September 3, 2016.
-
- Sundaram R, Shanthi P, Sachdanandam P. Effect of iridoid glucoside on plasma lipid profile, tissue fatty acid changes, inflammatory cytokines, and GLUT4 expression in skeletal muscle of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2013;380(1-2):43–55. doi: 10.1007/s11010-013-1656-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R56 DK108921/DK/NIDDK NIH HHS/United States
- R01 EY020582/EY/NEI NIH HHS/United States
- R24 DK082841/DK/NIDDK NIH HHS/United States
- R01 DK108921/DK/NIDDK NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U2C DK110768/DK/NIDDK NIH HHS/United States
- K08 DK089117/DK/NIDDK NIH HHS/United States
- DP3 DK094292/DK/NIDDK NIH HHS/United States
- K01 HL124050/HL/NHLBI NIH HHS/United States
- R03 DK106304/DK/NIDDK NIH HHS/United States
- R01 DK059997/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- U01 DK057329/DK/NIDDK NIH HHS/United States
- I01 BX002367/BX/BLRD VA/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- DP3 DK094352/DK/NIDDK NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- R01 GM114029/GM/NIGMS NIH HHS/United States
- U24 DK097153/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

