Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery
- PMID: 27699254
- PMCID: PMC5033865
- DOI: 10.1172/jci.insight.85311
Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery
Abstract
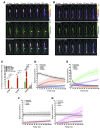
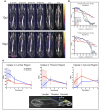
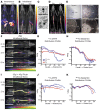
The intrathecal (IT) dosing route offers a seemingly obvious solution for delivering drugs directly to the central nervous system. However, gaps in understanding drug molecule behavior within the anatomically and kinetically unique environment of the mammalian IT space have impeded the establishment of pharmacokinetic principles for optimizing regional drug exposure along the neuraxis. Here, we have utilized high-resolution single-photon emission tomography with X-ray computed tomography to study the behavior of multiple molecular imaging tracers following an IT bolus injection, with supporting histology, autoradiography, block-face tomography, and MRI. Using simultaneous dual-isotope imaging, we demonstrate that the regional CNS tissue exposure of molecules with varying chemical properties is affected by IT space anatomy, cerebrospinal fluid (CSF) dynamics, CSF clearance routes, and the location and volume of the injected bolus. These imaging approaches can be used across species to optimize the safety and efficacy of IT drug therapy for neurological disorders.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

