Ex Vivo Methods for Informing Computational Models of the Mitral Valve
- PMID: 27699507
- PMCID: PMC5300906
- DOI: 10.1007/s10439-016-1734-z
Ex Vivo Methods for Informing Computational Models of the Mitral Valve
Abstract
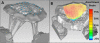
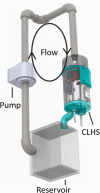
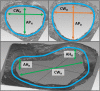
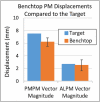
Computational modeling of the mitral valve (MV) has potential applications for determining optimal MV repair techniques and risk of recurrent mitral regurgitation. Two key concerns for informing these models are (1) sensitivity of model performance to the accuracy of the input geometry, and, (2) acquisition of comprehensive data sets against which the simulation can be validated across clinically relevant geometries. Addressing the first concern, ex vivo micro-computed tomography (microCT) was used to image MVs at high resolution (~40 micron voxel size). Because MVs distorted substantially during static imaging, glutaraldehyde fixation was used prior to microCT. After fixation, MV leaflet distortions were significantly smaller (p < 0.005), and detail of the chordal tree was appreciably greater. Addressing the second concern, a left heart simulator was designed to reproduce MV geometric perturbations seen in vivo in functional mitral regurgitation and after subsequent repair, and maintain compatibility with microCT. By permuting individual excised ovine MVs (n = 5) through each state (healthy, diseased and repaired), and imaging with microCT in each state, a comprehensive data set was produced. Using this data set, work is ongoing to construct and validate high-fidelity MV biomechanical models. These models will seek to link MV function across clinically relevant states.
Keywords: Cardiovascular; Imaging; Micro-computed tomography; Mitral regurgitation; Mitral repair; Simulation.
Figures





References
-
- Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D'Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O'Gara PT, Michler RE, Kron IL, Ctsn Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. - PMC - PubMed
-
- Borger MA, Alam A, Murphy PM, Doenst T, David TE. Chronic ischemic mitral regurgitation: repair, replace or rethink? Ann Thorac Surg. 2006;81:1153–1161. - PubMed
-
- Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, Chatellier G, Chauvaud S, Fabiani JN, Carpentier A. Very long-term results (more than 20 years) of valve repair with carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104:I8–11. - PubMed
-
- Carpentier A, Adams DH, Filsoufi F. Carpentier's reconstructive valve surgery. Elsevier Health Sciences. 2011
-
- Daimon M, Saracino G, Fukuda S, Koyama Y, Kwan J, Song JM, Agler DA, Gillinov AM, Thomas JD, Shiota T. Dynamic change of mitral annular geometry and motion in ischemic mitral regurgitation assessed by a computerized 3D echo method. Echocardiography. 2010;27:1069–1077. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

