Non-invasive Vagus Nerve Stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: an open-label study
- PMID: 27699586
- PMCID: PMC5047863
- DOI: 10.1186/s10194-016-0684-z
Non-invasive Vagus Nerve Stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: an open-label study
Abstract
Background: Menstrual migraine and menstrually related migraine attacks are typically longer, more disabling, and less responsive to medications than non-menstrual attacks. The aim of this study was to evaluate the efficacy, safety, and tolerability of non-invasive vagus nerve stimulation for the prophylactic treatment of menstrual migraine/menstrually related migraine.
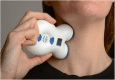
Methods: Fifty-six enrolled subjects (menstrual migraine, 9 %; menstrually related migraine, 91 %), 33 (59 %) of whom were receiving other prophylactic therapies, entered a 12-week baseline period. Fifty-one subjects subsequently entered a 12-week treatment period to receive open-label prophylactic non-invasive vagus nerve stimulation adjunctively (31/51; 61 %) or as monotherapy (20/51; 39 %) on day -3 before estimated onset of menses through day +3 after the end of menses.
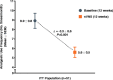
Results: The number of menstrual migraine/menstrually related migraine days per month was significantly reduced from baseline (mean ± standard error, 7.2 ± 0.7 days) to the end of treatment (mean ± standard error, 4.7 ± 0.5 days; P < 0.001) (primary end point). Of all subjects, 39 % (95 % confidence interval: 26 %, 54 %) (20/51) had a ≥ 50 % reduction (secondary end point). For the other secondary end points, clinically meaningful reductions in analgesic use (mean change ± standard error, -3.3 ± 0.6 times per month; P < 0.001), 6-item Headache Impact Test score (mean change ± standard error, -3.1 ± 0.7; P < 0.001), and Migraine Disability Assessment score (mean change ± standard error, -11.9 ± 3.4; P < 0.001) were observed, along with a modest reduction in pain intensity (mean change ± standard error, -0.5 ± 0.2; P = 0.002). There were no safety/tolerability concerns.
Conclusions: These findings suggest that non-invasive vagus nerve stimulation is an effective treatment that reduces the number of menstrual migraine/menstrually related migraine days and analgesic use without safety/tolerability concerns in subjects with menstrual migraine/menstrually related migraine. Randomised controlled studies are warranted.
Keywords: Menstrual migraine; Menstrually related migraine; Prophylactic treatment; Vagus nerve.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

