Protein Kinase C Signaling in Adenoviral Infection
- PMID: 27700064
- PMCID: PMC5808905
- DOI: 10.1021/acs.biochem.6b00858
Protein Kinase C Signaling in Adenoviral Infection
Abstract
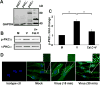
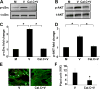
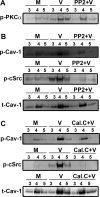
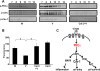
Activation of protein kinase C (PKC), a serine/threonine protein kinase, ubiquitously influences cellular signal transduction and has been shown to play a role in viral entry. In this study, we explored a role for PKC in human adenovirus type 37 infection of primary human corneal fibroblasts, a major target cell for infection. We sought evidence for an interaction between PKC activation and two potential downstream targets: cSrc kinase, shown previously to play a critical role in adenovirus signaling in these cells, and caveolin-1, reported earlier to be important to entry of adenovirus type 37. Infection of fibroblasts increased PKCα phosphorylation and translocation of PKCα from the cytosol to caveolin-1 containing vesicles. Virus-induced phosphorylation of both cSrc and AKT was abolished in cell lysates pretreated with calphostin C, a chemical inhibitor of PKC. Inhibition of PKC also reduced virus associated phosphorylation of caveolin-1, while inhibition of cSrc by the chemical inhibitor PP2 reduced only caveolin-1 phosphorylation, but not PKCα phosphorylation, in lipid rafts. These results suggest a role for PKCα upstream to both cSrc and caveolin-1. Phosphorylated PKCα was found in the same endosomal fractions as phosphorylated cSrc, and PKCα was present to a greater degree in caveolin-1 pull downs from virus infected than mock infected cell lysates. Calphostin C also reduced early viral gene expression, indicating that PKCα activity may be required for viral entry. PKCα plays a central role in adenovirus infection of corneal fibroblasts and regulation of downstream molecules, including the important lipid raft component caveolin-1.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

