Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay
- PMID: 27701109
- PMCID: PMC5488708
- DOI: 10.1126/science.aaf7783
Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay
Abstract
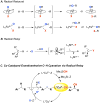
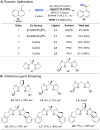
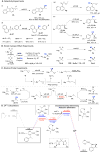
Direct methods for stereoselective functionalization of sp3-hybridized carbon-hydrogen [C(sp3)-H] bonds in complex organic molecules could facilitate much more efficient preparation of therapeutics and agrochemicals. Here, we report a copper-catalyzed radical relay pathway for enantioselective conversion of benzylic C-H bonds into benzylic nitriles. Hydrogen-atom abstraction affords an achiral benzylic radical that undergoes asymmetric C(sp3)-CN bond formation upon reaction with a chiral copper catalyst. The reactions proceed efficiently at room temperature with the benzylic substrate as limiting reagent, exhibit broad substrate scope with high enantioselectivity (typically 90 to 99% enantiomeric excess), and afford products that are key precursors to important bioactive molecules. Mechanistic studies provide evidence for diffusible organic radicals and highlight the difference between these reactions and C-H oxidations mediated by enzymes and other catalysts that operate via radical rebound pathways.
Copyright © 2016, American Association for the Advancement of Science.
Figures

References
-
- Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52:6752–6756. - PubMed
-
- Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev. 2004;104:3947–3980. - PubMed
-
- Butler A, Sandy M. Mechanistic considerations of halogenating enzymes. Nature. 2009;460:848–854. - PubMed
-
- Chen MS, White MC. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

