Ligand-accelerated enantioselective methylene C(sp3)-H bond activation
- PMID: 27701111
- PMCID: PMC5516954
- DOI: 10.1126/science.aaf4434
Ligand-accelerated enantioselective methylene C(sp3)-H bond activation
Abstract
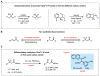
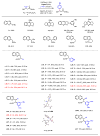
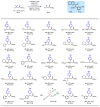
Effective differentiation of prochiral carbon-hydrogen (C-H) bonds on a single methylene carbon via asymmetric metal insertion remains a challenge. Here, we report the discovery of chiral acetyl-protected aminoethyl quinoline ligands that enable asymmetric palladium insertion into prochiral C-H bonds on a single methylene carbon center. We apply these palladium complexes to catalytic enantioselective functionalization of β-methylene C-H bonds in aliphatic amides. Using bidentate ligands to accelerate C-H activation of otherwise unreactive monodentate substrates is crucial for outcompeting the background reaction driven by substrate-directed cyclopalladation, thereby avoiding erosion of enantioselectivity. The potential of ligand acceleration in C-H activation is also demonstrated by enantioselective β-C-H arylation of simple carboxylic acids without installing directing groups.
Copyright © 2016, American Association for the Advancement of Science.
Figures

References
-
- Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem Soc Rev. 2009;38:3242–3272. - PubMed
-
- Doyle MP, Duffy R, Ratnikov M, Zhou L. Catalytic carbene insertion into C–H bonds. Chem Rev. 2010;110:704–724. - PubMed
-
- Reddy RP, Davies HML. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org Lett. 2006;8:5013–5016. - PubMed
-
- Liang C, Robert-Peillard F, Fruit C, Muller P, Dodd RH, Dauban P. Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew Chem Int Ed. 2006;45:4641–4644. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

