Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients
- PMID: 27701388
- PMCID: PMC5104887
- DOI: 10.1038/bjc.2016.308
Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients
Abstract
Background: Radiological assessment of response to checkpoint inhibitors remains imperfect. We evaluated individual lesion and inter-patient response by response evaluation (RECIST) 1.1, immune-related response criteria (irRC), CHOI and modified CHOI (mCHOI) and correlated response with overall survival (OS).
Methods: Thirty-seven patients with 567 measurable lesions treated with pembrolizumab in the Keynote 001 trial were studied. Association of response with OS was determined.
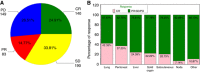
Results: Response varied according to site; lung lesions had the highest rate of complete response (69 out of 163 (42%) vs other sites 71 out of 404 (18%), P<0.0001). Delayed response post first scan was seen in 2 out of 37 (5%) deemed progressive (PD) by RECIST and 2 out of 14 (14%) deemed PD by irRC. Modified CHOI criteria showed response of 38% (14 out of 37). Change in tumour size and density on first follow-up assessment was associated with OS with each 1000 mm2 increase in tumour size from baseline increasing the hazard of dying by 25.9% (HR=1.259, (95% CI=1.116-1.420), P=0.0002). Similarly, each 20HU increase in density increased the HR by 15% (HR=1.15, (95% CI 1.045-1.260), P=0.004). Response defined by any criteria had superior OS (CHOI P=0.0084; mCHOI P=0.0183; irRC P<0.0001 and RECIST P=0.0003).
Conclusions: Response by any criterion was prognostic. Novel patterns of response and changes on treatment in tumour density suggest complex anti-tumour responses to immunotherapy.
Conflict of interest statement
DH serves on Advisory Boards for Roche, Merck, GlaxoSmithKline, Bristol-Myers Squibb and Novartis. CG has served on advisory boards for Novartis and Bristol-Myers Squibb. MB serves on Advisory Boards for Merck, Novartis, Bristol-Myeers Squibb and Immunovaccine. LK is currently an employee of AstraZeneca.
Figures

References
-
- Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA (2004) CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 183(6): 1619–1628. - PubMed
-
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25(13): 1753–1759. - PubMed
-
- Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer immunotherapy. Science 342(6165): 1432–1433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

