Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect
- PMID: 27701411
- PMCID: PMC5049772
- DOI: 10.1371/journal.pbio.1002564
Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect
Erratum in
-
Correction: Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect.PLoS Biol. 2016 Dec 29;14(12):e1002589. doi: 10.1371/journal.pbio.1002589. eCollection 2016 Dec. PLoS Biol. 2016. PMID: 28033324 Free PMC article.
Abstract
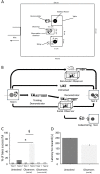
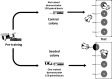
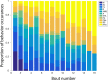
Social insects make elaborate use of simple mechanisms to achieve seemingly complex behavior and may thus provide a unique resource to discover the basic cognitive elements required for culture, i.e., group-specific behaviors that spread from "innovators" to others in the group via social learning. We first explored whether bumblebees can learn a nonnatural object manipulation task by using string pulling to access a reward that was presented out of reach. Only a small minority "innovated" and solved the task spontaneously, but most bees were able to learn to pull a string when trained in a stepwise manner. In addition, naïve bees learnt the task by observing a trained demonstrator from a distance. Learning the behavior relied on a combination of simple associative mechanisms and trial-and-error learning and did not require "insight": naïve bees failed a "coiled-string experiment," in which they did not receive instant visual feedback of the target moving closer when tugging on the string. In cultural diffusion experiments, the skill spread rapidly from a single knowledgeable individual to the majority of a colony's foragers. We observed that there were several sequential sets ("generations") of learners, so that previously naïve observers could first acquire the technique by interacting with skilled individuals and, subsequently, themselves become demonstrators for the next "generation" of learners, so that the longevity of the skill in the population could outlast the lives of informed foragers. This suggests that, so long as animals have a basic toolkit of associative and motor learning processes, the key ingredients for the cultural spread of unusual skills are already in place and do not require sophisticated cognition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Heyes CM, Galef BG. Social Learning In Animals: The Roots of Culture. Elsevier; 1996.
-
- Breed MD, Moore J. Encyclopedia of Animal Behavior 1st ed London: Academic Press; 2010.
-
- Hopper LM, Whiten A. The comparative and evolutionary psychology of social learning and culture The Oxford Handbook of Comparative Evolutionary Psychology. Oxford University Press. J. Vonk & T. Shackelford; 2012. pp. 451–473.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

