Fine-tuning vascular fate during endothelial-mesenchymal transition
- PMID: 27701751
- PMCID: PMC5164846
- DOI: 10.1002/path.4814
Fine-tuning vascular fate during endothelial-mesenchymal transition
Abstract
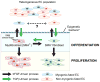
In the heart and other organs, endothelial-mesenchymal transition (EndMT) has emerged as an important developmental process that involves coordinated migration, differentiation, and proliferation of the endothelium. In multiple disease states including cancer angiogenesis and cardiovascular disease, the processes that regulate EndMT are recapitulated, albeit in an uncoordinated and dysregulated manner. Members of the transforming growth factor beta (TGFβ) superfamily are well known to impart cellular plasticity during EndMT by the timely activation (or repression) of transcription factors and miRNAs in addition to epigenetic regulation of gene expression. On the other hand, fibroblast growth factors (FGFs) are reported to augment or oppose TGFβ-driven EndMT in specific contexts. Here, we have synthesized the currently understood roles of TGFβ and FGF signalling during EndMT and have provided a new, comprehensive paradigm that delineates how an autocrine and paracrine TGFβ/FGF axis coordinates endothelial cell specification and plasticity. We also provide new guidelines and nomenclature that considers factors such as endothelial cell heterogeneity to better define EndMT across different vascular beds. This perspective should therefore help to clarify why TGFβ and FGF can both cooperate with or oppose one another during the complex process of EndMT in both health and disease. Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: TGFβ; bFGF; cardiovascular disease; endothelial heterogeneity; endothelial plasticity; endothelial-to-mesenchymal transition; tumour microenvironment.
Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
No conflicts of interest were disclosed
Figures
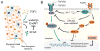

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

