Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies
- PMID: 27701752
- PMCID: PMC6457998
- DOI: 10.1002/14651858.CD002827.pub4
Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies
Abstract
Background: Serum monoclonal anti-myelin-associated glycoprotein (anti-MAG) antibodies may be pathogenic in some people with immunoglobulin M (IgM) paraprotein and demyelinating neuropathy. Immunotherapies aimed at reducing the level of these antibodies might be expected to be beneficial. This is an update of a review first published in 2003 and previously updated in 2006 and 2012.
Objectives: To assess the effects of immunotherapy for IgM anti-MAG paraprotein-associated demyelinating peripheral neuropathy.
Search methods: On 1 February 2016 we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase for randomised controlled trials (RCTs). We also checked trials registers and bibliographies, and contacted authors and experts in the field.
Selection criteria: We included randomised controlled trials (RCTs) or quasi-RCTs involving participants of any age treated with any type of immunotherapy for anti-MAG antibody-associated demyelinating peripheral neuropathy with monoclonal gammopathy of undetermined significance and of any severity.Our primary outcome measures were numbers of participants improved in disability assessed with either or both of the Neuropathy Impairment Scale (NIS) or the modified Rankin Scale (mRS) at six months after randomisation. Secondary outcome measures were: mean improvement in disability, assessed with either the NIS or the mRS, 12 months after randomisation; change in impairment as measured by improvement in the 10-metre walk time, change in a validated linear disability measure such as the Rasch-built Overall Disability Scale (R-ODS) at six and 12 months after randomisation, change in subjective clinical scores and electrophysiological parameters at six and 12 months after randomisation; change in serum IgM paraprotein concentration or anti-MAG antibody titre at six months after randomisation; and adverse effects of treatments.
Data collection and analysis: We followed standard methodological procedures expected by Cochrane.
Main results: We identified eight eligible trials (236 participants), which tested intravenous immunoglobulin (IVIg), interferon alfa-2a, plasma exchange, cyclophosphamide and steroids, and rituximab. Two trials of IVIg (22 and 11 participants, including 20 with antibodies against MAG), had comparable interventions and outcomes, but both were short-term trials. We also included two trials of rituximab with comparable interventions and outcomes.There were very few clinical or statistically significant benefits of the treatments used on the outcomes predefined for this review, but not all the predefined outcomes were used in every included trial and more responsive outcomes are being developed. A well-performed trial of IVIg, which was at low risk of bias, showed a statistical benefit in terms of improvement in mRS at two weeks and 10-metre walk time at four weeks, but these short-term outcomes are of questionable clinical significance. Cyclophosphamide failed to show any benefit in the single trial's primary outcome, and showed a barely significant benefit in the primary outcome specified here, but some toxic adverse events were identified.Two trials of rituximab (80 participants) have been published, one of which (26 participants) was at high risk of bias. In the meta-analysis, although the data are of low quality, rituximab is beneficial in improving disability scales (Inflammatory Neuropathy Cause and Treatment (INCAT) improved at eight to 12 months (risk ratio (RR) 3.51, 95% confidence interval (CI) 1.30 to 9.45; 73 participants)) and significantly more participants improve in the global impression of change score (RR 1.86, 95% CI 1.27 to 2.71; 70 participants). Other measures did not improve significantly, but wide CIs do not preclude some effect. Reported adverse effects of rituximab were few, and mostly minor.There were few serious adverse events in the other trials.
Authors' conclusions: There is inadequate reliable evidence from trials of immunotherapies in anti-MAG paraproteinaemic neuropathy to form an evidence base supporting any particular immunotherapy treatment. IVIg has a statistically but probably not clinically significant benefit in the short term. The meta-analysis of two trials of rituximab provides, however, low-quality evidence of a benefit from this agent. The conclusions of this meta-analysis await confirmation, as one of the two included studies is of very low quality. We require large well-designed randomised trials of at least 12 months' duration to assess existing or novel therapies, preferably employing unified, consistent, well-designed, responsive, and valid outcome measures.
Conflict of interest statement
MPTL has published a pilot study of the use of fludarabine in IgM‐associated paraproteinaemic neuropathy (Wilson 1999) and was a blinded investigator in Comi 2002. He has received honoraria for consultation from Baxter Pharmaceuticals, CSL Behring, UCB and LFB and a travel support grant from CSL Behring and Grifols, all manufacturers of IVIg. He was a blinded Site Investigator on the FORCIDP study (fingolimod in CIDP (Novartis) who in August 2015 purchased ofatumumab (not included in this review). He is Joint Co‐ordinating Editor of Cochrane Neuromuscular. He did not have a role in editorial assessment or the decision to publish this updated review.
EN‐O has received honoraria from CSL Behring (Italy and USA), UCB (UK), and Kedrion (Italy) for Scientific Advisory Board participation and from Novartis (Switzerland) for being part of a Steering Committee for a trial with fingolimod on CIDP. He received compensation from Baxalta, Italy for preparing a teaching course on Multifocal Motor Neuropathy. He received compensation for lectures from Kedrion, Italy, CSL Behring, Italy and Baxter, USA. He received support for Meeting attendance from CSL Behring, Italy and Kedrion Italy. He has published a study of long‐term prognosis of IgM neuropathies and their immunotherapy (Nobile‐Orazio 2000) and was a member of the BIOMED INCAT Group which conducted the Comi 2002 study. He was the principal investigator in a RCT sponsored by Kedrion comparing the efficacy of intravenous corticosteroids with IVIg in CIDP. He is the Principal Investigator of a trial on IVIg in CIDP sponsored by LFB, France. He states that no part of the above‐mentioned financial support had any influence on his work in preparing this review.
Both review authors were investigators in Comi 2002. The Cochrane Neuromuscular Managing Editor carried out an independent risk of bias assessment and data extraction of outcome data for this study, and checked these against the data entered in the review.
This review was first published in 2003. One new trial, of rituximab, has been added at this update. Both review authors have declared financial remuneration for consultancy work relating to IVIg. The most recent trial of IVIg was published in 2002, the conclusion of the review being that the short‐term effects of this intervention are of doubtful clinical significance in this condition, a conclusion unchanged at this update.
Although the authorship of this updated review does not fully comply with the current Conflict of Interests policy, it did at the time of original publication. This has been discussed with the Funding Arbiters who have agreed that there is a low risk of significant bias resulting from the declared financial and academic interests.
Figures










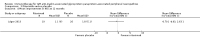













Update of
-
Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies.Cochrane Database Syst Rev. 2012 May 16;(5):CD002827. doi: 10.1002/14651858.CD002827.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Oct 04;10:CD002827. doi: 10.1002/14651858.CD002827.pub4. PMID: 22592686 Updated.
References
References to studies included in this review
Comi 2002 {published data only}
-
- Comi G, Roveri L, Swan A, Willison H, Bojar M, Illa I, et al. Inflammatory Neuropathy Cause And Treatment (INCAT) Group. A randomised controlled trial of intravenous immunoglobulin in IgM paraprotein associated demyelinating neuropathy. Journal of Neurology 2002;249(10):1370‐7. [PUBMED: 12382151] - PubMed
Dalakas 1996 {published data only}
-
- Dalakas MC, Quarles RH, Farrer RG, Dambrosia J, Soueidan S, Stein DP, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Annals of Neurology 1996;40(5):792‐5. [PUBMED: 8957021] - PubMed
Dalakas 2009 {published data only}
-
- Dalakas MC, Rakocevic G, Salajegheh M, Dambrosia JM, Hahn AF, Raju R, et al. Placebo‐controlled trial of rituximab in IgM anti–myelin‐associated glycoprotein antibody demyelinating neuropathy. Annals of Neurology 2009;65(3):286–93. [PUBMED: 19334068] - PubMed
Léger 2013 {published data only}
Mariette 1997 {published data only}
-
- Mariette X, Chastang C, Clavelou P, Louboutin J‐P, Leger JM, Brouet JC. A randomised clinical trial comparing interferon‐alpha and intravenous immunoglobulin in polyneuropathy associated with monoclonal IgM. The IgM‐associated Polyneuropathy Study Group. Journal of Neurology, Neurosurgery and Psychiatry 1997;63(1):28‐34. [PUBMED: 9221964] - PMC - PubMed
Mariette 2000 {published data only}
-
- Mariette X, Brouet JC, Chevret S, Leger JM, Clavelou P, Pouget J, et al. A randomized double blind trial versus placebo does not confirm the benefit of alpha‐interferon in polyneuropathy associated with monoclonal IgM. Journal of Neurology, Neurosurgery and Psychiatry 2000;69(2):279‐80. [PUBMED: 10960293] - PMC - PubMed
Niermeijer 2007 {published data only}
-
- Niermeijer JMF, Eurelings M, Van der Linden MW, Lokhorst HM, Franssen H, Fischer K, et al. Intermittent cyclophosphamide with prednisone versus placebo for polyneuropathy with IgM monoclonal gammopathy. Neurology 2007;69(1):50‐9. [PUBMED: 17606880] - PubMed
Oksenhendler 1995 {published data only}
-
- Oksenhendler E, Chevret S, Leger JM, Louboutin JP, Bussel A, Brouet JC. Plasma exchange and chlorambucil in polyneuropathy associated with monoclonal IgM gammopathy. IgM‐associated Polyneuropathy Study Group. Journal of Neurology, Neurosurgery and Psychiatry 1995;59(3):243‐7. [PUBMED: 7673949] - PMC - PubMed
References to studies excluded from this review
Barohn 2005 {published data only}
-
- Barohn RJ, Rashid I, McVey AL, Herbelin L, Skikne B, Saperstein DS. Rituximab for the treatment of IgM associated polyneuropathies. Journal of the Peripheral Nervous System 2005;10 Suppl 1:4.
Benedetti 2005 {published data only}
-
- Benedetti L, Gobbi M, Ghiglione E, Vigo T, Carpo M, Cocito D, et al. Rituximab in anti‐MAG polyneuropathy. Journal of the Peripheral Nervous System 2005;10(Suppl 1):5. - PubMed
Benedetti 2007 {published data only}
-
- Benedetti B, Briani C, Grnadis M, Vigo T, Gobbi M, Ghiglione E, et al. Predictors of response to rituximab in patients with neuropathy and anti‐myelin associated glycoprotein immunoglobulin M. Journal of the Peripheral Nervous System 2007;12(2):102‐7. - PubMed
Benedetti 2008 {published data only}
-
- Benedetti L, Briani C, Franciotta D, Carpo M, Padua L, Zara G, et al. Long‐term effect of rituximab in anti‐MAG polyneuropathy. Neurology 2008;71(21):1742‐4. - PubMed
Canavan 2002 {published data only}
-
- Canavan JB, Moroney JT, Keogan MT, Hardiman O. Rituximab and IgM autoantibody‐associated peripheral neuropathy: The Irish experience. Neurology 2002;Suppl 3:A233.
Dyck 1991 {published data only}
-
- Dyck PJ, Low PA, Windebank AJ, Jaradeh SS, Gosselin S, Bourque P, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. New England Journal of Medicine 1991;325(21):1482‐6. [MEDLINE: ] - PubMed
Goldfarb 2005 {published data only}
-
- Goldfarb AR, Weimer LH, Brannigan TH 3rd. Rituximab treatment of an IgM monoclonal autonomic and sensory neuropathy. Muscle & Nerve 2005;31(4):510‐5. - PubMed
Gorson 2004 {published data only}
-
- Gorson KC, Amato AA, Ropper AH. Efficacy of mycophenolate mofetil in patients with chronic immune demyelinating polyneuropathy. Neurology 2004;63(4):715‐7. - PubMed
Gorson 2007 {published data only}
-
- Gorson KC, Natarajan N, Ropper AH, Weinstein R. Rituximab treatment in patients with IVIg‐dependent immune polyneuropathy: a prospective pilot trial. Muscle & Nerve 2007;35(1):66‐9. - PubMed
Hamidou 2005 {published data only}
-
- Hamidou MA, Belizna C, Wiertlewsky S, Audrain M, Biron C, Grolleau J, et al. Intravenous cyclophosphamide in refractory polyneuropathy associated with IgM monoclonal gammopathy: an uncontrolled open trial. American Journal of Medicine 2005;118(4):426‐30. - PubMed
Kilidireas 2006 {published data only}
-
- Kilidireas C, Anagnostopoulos A, Karandreas N, Mouselimi L, Dimopoulos MA. Rituximab therapy in monoclonal IgM‐related neuropathies. Leukemia & Lymphoma 2006;47(5):859‐64. - PubMed
Latov 1999 {published data only}
-
- Latov N, Sherman WH. Therapy of neuropathy associated with anti‐MAG IgM monoclonal gammopathy with Rituxan (abstract). Neurology 1999;52 Suppl 2:A551.
Niermeijer 2006 {published data only}
-
- Niermeijer JMF, Eurelings M, Lokhorst H, Franssen H, Fijnheer R, Wokke JHJ, et al. Neurologic and hematologic response to fludarabine treatment in IgM MGUS polyneuropathy. Neurology 2006;67(11):2076‐9. - PubMed
Niermeijer 2009 {published data only}
-
- Niermeijer JM, Eurelings M, Lokhorst HL, Pol WL, Franssen H, Wokke JH, et al. Rituximab for polyneuropathy with IgM monoclonal gammopathy. Journal of Neurology, Neurosurgery and Psychiatry 2009;80(9):1036‐9. - PubMed
Pestronk 2003 {published data only}
Renaud 2003 {published data only}
-
- Renaud S, Gregor M, Fuhr P, Lorenz D, Deuschl G, Gratwohl A, et al. Rituximab in the treatment of polyneuropathy associated with anti‐MAG antibodies. Muscle & Nerve 2003;27(5):611‐5. - PubMed
Renaud 2006 {published data only}
-
- Renaud S, Fuhr P, Gregor M, Schweikert K, Lorenz D, Daniels C, et al. High‐dose rituximab and anti‐MAG‐associated polyneuropathy. Neurology 2006;66(5):742‐4. - PubMed
Sghirlanzoni 2000 {published data only}
-
- Sghirlanzoni A, Solari A, Ciano C, Mariotti C, Fallica E, Pareyson D. Chronic inflammatory demyelinating polyradiculoneuropathy: long‐term course and treatment of 60 patients. Neurological Sciences 2000;21(1):31‐7. - PubMed
Smith 2011 {published data only}
-
- Smith BE, Suarez GA, Witzig TE, Stevens JC, Bosch EP, Ross MA, et al. A phase II trial of rituximab for peripheral neuropathy associated with monoclonal gammopathy of undetermined significance (MGUS). Journal of the Peripheral Nervous System 2011;16(Suppl s3):130.
Additional references
Ad Hoc 1991
-
- Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for the diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology 1991;41(5):617‐8. - PubMed
Andres 2001
-
- Andres E, Vinzio S, Maloisel F, Carre S, Perrin AE, Goichot B, et al. Autoimmune peripheral neuropathies with anti‐MAG antibodies and hematological disorders. Five cases [Neuropathies péripheriques auto‐immunes a anticorps anti‐MAG et hémopathies. À propos de 5 observations]. Annales de Médecine Interne 2001;152(3):147‐51. - PubMed
Bamford 1989
-
- Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20(6):828. - PubMed
Bland 1985
-
- Bland JH, Gennari FJ, Erschler WB, Latov N. IgMk monoclonal antibody directed against peripheral nerve myelin; clinical peripheral neuropathy and long‐term rheumatic disease. Journal of Rheumatology 1985;12(6):1200. - PubMed
Blume 1995
-
- Blume G, Pestronk A, Goodnough LT. Anti‐MAG antibody‐associated polyneuropathies: improvement following immunotherapy with monthly plasma exchange and IV cyclophosphamide. Neurology 1995;45(8):1577‐80. - PubMed
Broglio 2005
-
- Broglio L, Lauria G. Worsening after rituximab treatment in anti‐MAG neuropathy. Muscle & Nerve 2005;32(3):378‐9. - PubMed
Chassande 1998
-
- Chassande B, Leger JM, Younes‐Chennoufi AB, Bengoufa D, Maisonobe T, Bouche P, et al. Peripheral neuropathy associated with IgM monoclonal gammopathy: correlations between M‐protein antibody activity and clinical/electrophysiological features in 40 cases. Muscle & Nerve 1998;21(6):55‐62. - PubMed
Cook 1990
-
- Cook D, Dalakas MC, Galdi A, Biondi D, Porter H. High‐dose intravenous immunoglobulin in the treatment of demyelinating neuropathy associated with monoclonal gammopathy [see comments]. Neurology 1990;40:212‐4. [MEDLINE: ] - PubMed
Dalakas 1981
-
- Dalakas MC, Engel WK. Polyneuropathy with monoclonal gammopathy: studies of 11 patients. Annals of Neurology 1981;10(1):45‐52. [MEDLINE: ] - PubMed
Delmont 2010
-
- Delmont E, Jeandel PY, Hubert AM, Marcq L, Boucraut J, Desnuelle C. Successful treatment with rituximab of one patient with CANOMAD neuropathy. Journal of Neurology 2010;257(4):655‐7. - PubMed
Donofrio 1989
-
- Donofrio PD, Kelly JJ Jr. AAEE case report #17: Peripheral neuropathy in monoclonal gammopathy of undetermined significance. Muscle & Nerve 1989;12(1):1‐8. - PubMed
Dubas 1987
-
- Dubas F, Pouplard‐Barthelaix A, Delestre F, Emile J. Polyneuropathies with IgM monoclonal gammopathy. 12 cases. Revue Neurologique 1987;143(10):670‐83. - PubMed
Dyck 1980
-
- Dyck PJ, Sherman WR, Hallcher LM, Service FJ, O'Brien PC, Grina LA, et al. Human diabetic endoneurial sorbitol, fructose, and myo‐inositol related to sural nerve morphometry. Annals of Neurology 1980;8(6):590‐6. - PubMed
Dyck 2005
-
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: Elsevier Saunders, 2005:1031‐53.
Ellie 1996
-
- Ellie E, Vital A, Steck A, Boiron J‐M, Vital C, Julien J. Neuropathy associated with 'benign' anti‐myelin‐associated glycoprotein IgM gammopathy: clinical, immunological, neurophysiological and pathological findings and response to treatment in 33 cases. Journal of Neurology 1996;243(1):34‐43. - PubMed
Ernerudh 1986
-
- Ernerudh J, Brodtkorb E, Olsson T, Vedeler CA, Nyland H, Berlin G. Peripheral neuropathy and monoclonal IgM with antibody activity against peripheral nerve myelin; effect of plasma exchange. Journal of Neuroimmunology 1986;11(3):171‐8. - PubMed
Ernerudh 1992
Ferrari 1998
-
- Ferrari S, Morbin M, Nobile‐Orazio E, Musso A, Tomelleri G, Bertolasi L, et al. Antisulfatide polyneuropathy: antibody‐mediated complement attack on peripheral myelin. Acta Neuropathologica 1998;96(6):569‐74. - PubMed
Frayne 1985
-
- Frayne D, Starke RJ. Peripheral neuropathy with gammopathy responding to plasmapheresis. Clinical and Experimental Neurology 1985;21:195‐200. - PubMed
Ghosh 2002
-
- Ghosh A, Littlewood T, Donaghy M. Cladribine in the treatment of IgM paraproteinaemic polyneuropathy. Neurology 2002;59(8):1290‐1. - PubMed
Gironi 2006
-
- Gironi M, Saresella M, Ceresa L, Calvo M, Ferrante P, Merli F, et al. Clinical and immunological worsening in a patient affected with Waldenstrom macroglobulinemia and anti‐mag neuropathy after treatment with rituximab. Haematologica 2006;91(6 Suppl):ECR 17. - PubMed
Gono 2006
-
- Gono T, Matsuda M, Shimojima Y, Ishii W, Yamamoto K, Morita H, et al. Rituximab therapy in chronic inflammatory demyelinating polyradiculoneuropathy with anti‐SGPG IgM antibody. Journal of Clinical Neuroscience 2006;13(6):683‐7. - PubMed
Gorson 1997
-
- Gorson KC, Allam G, Ropper AH. Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology 1997;48(2):321‐8. - PubMed
Gorson 2001
-
- Gorson KC, Ropper AH, Weinberg DH, Weinstein R. Treatment experience in patients with anti‐myelin‐associated glycoprotein neuropathy. Muscle & Nerve 2001;24(6):778‐86. - PubMed
Gruson 2011
-
- Gruson B, Ghomari K, Beaumont M, Garidi R, Just A, Merle P, et al. Long‐term response to rituximab and fludarabine combination in IgM anti‐myelin‐associated glycoprotein neuropathy. Journal of the Peripheral Nervous System 2011;16(3):180‐5. - PubMed
Haas 1988
-
- Haas DC, Tatum AH. Plasmapheresis alleviates neuropathy accompanying IgM anti‐myelin‐associated glycoprotein paraproteinemia. Annals of Neurology 1988;23(4):394‐6. - PubMed
Hafler 1986
-
- Hafler DA, Johnson D, Kelly JJ, Panitch R, Kyle R, Weiner HL. Monoclonal gammopathy and neuropathy; myelin associated glycoprotein reactivity and clinical characteristics. Neurology 1986;36(1):75‐8. - PubMed
Hays 1988
-
- Hays AP, Lee SS, Latov N. Immune reactive C3d on the surface of myelin sheaths in neuropathy. Journal of Neuroimmunology 1988;18(3):231‐44. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoang‐Xuan 1993
-
- Hoang‐Xuan K, Leger JM, Ben Younes‐Chennoufi A, Saidi H, Bouche P, Baumann N, et al. Treatment of immune deficient neuropathies with intravenous polyvalent immunoglobulins. An open study of 16 cases. Revue Neurologique 1993;149(6‐7):385‐92. - PubMed
Hodgkinson 1990
Ilyas 2008
Kaku 1994
-
- Kaku DA, England JD, Sumner AJ. Distal accentuation of conduction slowing in polyneuropathy associated with antibodies to myelin‐associated glycoprotein and sulphated glucuronyl paragloboside. Brain 1994;117(Pt 5):941‐7. - PubMed
Kelly 1981
-
- Kelly JJ, Kyle RA, O'Brien PC, Dyck PJ. Prevalence of monoclonal protein in peripheral neuropathy. Neurology 1981;31(11):1480‐3. - PubMed
Kelly 1988
-
- Kelly JJ, Adelman LS, Berkman E, Bhan I. Polyneuropathies associated with IgM monoclonal gammopathies. Archives of Neurology 1988;45(12):1355‐9. - PubMed
Kelly 2006
-
- Kelly JJ. Chronic peripheral neuropathy responsive to rituximab. Reviews in Neurological Diseases 2006;3(2):78‐81. - PubMed
Latov 1980
-
- Latov N, Sherman WH, Nemni R, Galassi G, Shyong JS, Penn AS, et al. Plasma cell dyscrasia and peripheral neuropathy with a monoclonal antibody to peripheral nerve myelin. New England Journal of Medicine 1980;303(11):618‐21. - PubMed
Latov 1988
-
- Latov N, Hays AP, Sherman WH. Peripheral neuropathy and anti‐MAG antibodies. Critical Reviews in Neurobiology 1988;3(4):301‐32. - PubMed
Levine 1999
-
- Levine TD, Pestronk A. IgM antibody‐related polyneuropathies: B‐cell depletion chemotherapy using rituximab. Neurology 1999;52(8):1701‐4. - PubMed
Léger 1994
Meier 1984
-
- Meier C, Roberts K, Steck A, Hess C, Miloni E, Tschopp L. Polyneuropathy in Waldenstrom's macroglobulinaemia: reduction of endoneurial IgM‐deposits after treatment with chlorambucil and plasmapheresis. Acta Neuropathologica 1984;64(4):297‐307. - PubMed
Melmed 1983
-
- Melmed C, Frail D, Duncan I, Braun P, Danoff D, Finlayson M, et al. Peripheral neuropathy with IgM kappa monoclonal immunoglobulin directed against myelin‐associated glycoprotein. Neurology 1983;33(11):1397‐405. [MEDLINE: ] - PubMed
Merkies 1999
-
- Merkies IS, Schmitz PI, Samijn JP, Meche FG, Doorn PA. Fatigue in immune‐mediated polyneuropathies. European Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 1999;53(8):1648‐54. - PubMed
Merkies 2000
-
- Merkies IS, Schmitz PI, Meche FG, Doorn PA. Psychometric evaluation of a new sensory scale in immune‐mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 2000;54(4):943‐9. - PubMed
Monaco 1990
-
- Monaco S, Bonetti B, Ferrari S, Moretto G, Nardelli E, Tedesco F, et al. Complement‐mediated demyelination in patients with IgM monoclonal gammopathy and polyneuropathy. New England Journal of Medicine 1990;322(10):649‐52. [MEDLINE: ] - PubMed
Nicolas 2002
-
- Nicolas G, Maisonobe T, Forestier N, Léger JM, Bouche P. Proposed revised electrophysiological criteria for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve 2002;25(1):26‐30. - PubMed
Niemierko 1999
-
- Niemierko E, Weinstein R. Response of patients with IgM and IgA‐associated peripheral polyneuropathies to "off‐line" immunoadsorption treatment using the Prosorba Protein A column. Journal of Clinical Apheresis 1999;14(4):159‐62. - PubMed
Nobile‐Orazio 1988
-
- Nobile‐Orazio E, Baldini L, Barbieri S, Marmiroli P, Spagnol G, Francomano E, et al. Treatment of patients with neuropathy and anti‐MAG IgM M‐proteins. Annals of Neurology 1988;24(1):93‐7. - PubMed
Nobile‐Orazio 2000
-
- Nobile‐Orazio E, Meucci N, Baldini L, Troia A, Scarlato G. Long‐term prognosis of neuropathy associated with anti‐MAG IgM M‐proteins and its relationship to immune therapies. Brain 2000;123(Pt 4):710‐7. - PubMed
Noronha 2006
-
- Noronha V, Fynan TM, Duffy T. Flare in neuropathy following rituximab therapy for Waldenstrom’s macroglobulinemia. Journal of Clinical Oncology 2006;24(1):e3. - PubMed
Notermans 1996
-
- Notermans NC, Lokhorst HM, Franssen H, Graaf Y, Teunissen LL, Jennekens FG, et al. Intermittent cyclophosphamide and prednisone treatment of polyneuropathy associated with monoclonal gammopathy of undetermined significance. Neurology 1996;47(5):1227‐33. - PubMed
Notermans 1997
-
- Notermans NC, Vermeulen M, Lokhorst HM, Doorn PA, Berg LH, Teunissen LL, et al. Pulsed high‐dose dexamethasone treatment of polyneuropathy associated with monoclonal gammopathy. Journal of Neurology 1997;244(7):462‐3. - PubMed
Pouget 2000
-
- Pouget J, Azulay JPH, Mesure S, Attarian S. Improvement of sensory ataxia in anti‐MAG antibody associated polyneuropathy with interferon alpha: A posturographic study. Neurology 2000;54 Suppl 3:A47.
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rojas‐García 2003
-
- Rojas‐García R, Gallardo E, Andrés I, Luna N, Juarez C, Sánchez P, et al. Chronic neuropathy with IgM antiganglioside antibodies: lack of long term response to rituximab. Neurology 2003;61(12):1814–6. - PubMed
Rudnicki 1998
-
- Rudnicki SA, Harik SI, Dhodapkar M, Barlogie B, Eidelberg D. Nervous system dysfunction in Waldenstrom's macroglobulinemia: response to treatment. Neurology 1998;51(4):1210‐3. - PubMed
Senn 1993
-
- Senn SJ. Cross‐over Trials in Clinical Research. Chichester: Wiley, 1993.
Sherman 1984
Sherman 1994
-
- Sherman WH, Latov N, Lange DE, Hays RD, Younger DS. Fludarabine for IgM antibody‐mediated neuropathies. Annals of Neurology 1994;36:326‐7. [ISSN 0364‐5134]
Siciliano 1994
-
- Siciliano G, Moriconi L, Gianni G, Richieri E, Vignocchi MG, Rossi B. Selective techniques of apheresis in polyneuropathy associated with monoclonal gammopathy of undetermined significance. Acta Neurologica Scandanavica 1994;89(2):117‐22. - PubMed
Smith 1983
-
- Smith IS, Kahn SN, Lacey BW, King RHM, Eames RA, Whybrew DJ, et al. Chronic demyelinating neuropathy associated with benign IgM paraproteinaemia. Brain 1983;106(Pt 1):169‐95. - PubMed
Smith 1987
-
- Smith T, Sherman W, Olarte MR, Lovelace RE. Peripheral neuropathy associated with plasma cell dyscrasia: a clinical and electrophysiological follow‐up study. Acta Neurologica Scandanavica 1987;75(4):244‐8. - PubMed
Steck 1996
-
- Steck AJ, Kuntzer T. Anti‐glycoconjugate antibodies and dysglobulinemic or dysimmune peripheral neuropathies. Revue Neurologique 1996;152:400‐4. - PubMed
Stefansson 1983
-
- Stefansson K, Marton L, Antel JP, Wollmann RL, Roos RP, Chejfec G, et al. Neuropathy accompanying IgM lambda monoclonal gammopathy. Acta Neuropathologica 1983;59(4):255‐61. - PubMed
Tagawa 2001
-
- Tagawa Y, Yuki N, Ohnishi A, Hirata K, Hosokawa S. Parameters for monitoring treatment effects in CIDP with anti‐MAG/SGPG IgM antibody. Muscle & Nerve 2001;24(5):701‐4. - PubMed
Takatsu 1985
-
- Takatsu M, Hays AP, Latov N, Abrams GM, Nemni R, Sherman WH, et al. Immunofluorescence study of patients with neuropathy and IgM M proteins. Annals of Neurology 1985;18(2):173‐81. - PubMed
Tatum 1993
-
- Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti‐myelin‐associated glycoprotein. Annals of Neurology 1993;33(5):502‐6. - PubMed
Weide 2000
-
- Weide R, Heymanns J, Koppler H. The polyneuropathy associated with Waldenström's macroglobulinaemia can be treated effectively with chemotherapy and the anti‐CD20 monoclonal antibody rituximab. British Journal of Haematology 2000;109(4):838‐41. - PubMed
Willison 1988
-
- Willison HJ, Trapp BD, Bacher JD, Dalakas MC, Griffin JW, Quarles RH. Demyelination induced by intraneural injection of human antimyelin‐associated glycoprotein antibodies. Muscle & Nerve 1988;11(11):1169‐76. - PubMed
Willison 2001
-
- Willison HJ, O'Leary CP, Veitch J, Blumhardt LD, Busby M, Donaghy M, et al. The clinical and laboratory features of chronic sensory ataxic neuropathy with anti‐disialosyl IgM antibodies. Brain 2001;124(10):1968‐77. - PubMed
Wilson 1999
Yeung 1991
-
- Yeung KB, Thomas PK, King RH, Waddy H, Will RG, Hughes RAC, et al. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. Journal of Neurology 1991;238(7):383‐91. - PubMed
References to other published versions of this review
Lunn 2000
-
- Lunn MPT, Nobile‐Orazio E. Immunotherapy for IgM anti‐myelin‐associated glycoprotein paraprotein‐associated peripheral neuropathies. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002827] - DOI
Lunn 2003
Lunn 2006
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

