2014-2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type
- PMID: 27702768
- PMCID: PMC5146719
- DOI: 10.1093/cid/ciw635
2014-2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type
Abstract
Background: Circulating A/H3N2 influenza viruses drifted significantly after strain selection for the 2014-2015 vaccines. Also in 2014-2015, the Advisory Committee on Immunization Practices recommended preferential use of live attenuated influenza vaccine (LAIV) over inactivated influenza vaccine (IIV) among children aged 2-8 years.
Methods: Vaccine effectiveness (VE) across age groups and vaccine types was examined among outpatients with acute respiratory illness at 5 US sites using a test-negative design, that compared the odds of vaccination among reverse transcription polymerase chain reaction-confirmed influenza positives and negatives.
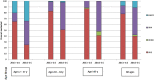
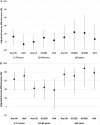
Results: Of 9311 enrollees with complete data, 7078 (76%) were influenza negative, 1840 (19.8%) were positive for influenza A (A/H3N2, n = 1817), and 395 (4.2%) were positive for influenza B (B/Yamagata, n = 340). The overall adjusted VE was 19% (95% confidence interval [CI], 10% to 27%) and was statistically significant in all age strata except those aged 18-64 years. The adjusted VE of 6% (95%CI, -5% to 17%) against A/H3N2-associated illness was not statistically significant, unlike VE for influenza B/Yamagata, which was 55% (95%CI, 43% to 65%). Among those aged 2-8 years, VE against A/H3N2 was 15% (95%CI, -16% to 38%) for IIV and -3% (CI, -50% to 29%) for LAIV; VE against B/Yamagata was 40% (95%CI, -20% to 70%) for IIV and 74% (95%CI, 25% to 91%) for LAIV.
Conclusions: The 2014-2015 influenza vaccines offered little protection against the predominant influenza A/H3N2 virus but were effective against influenza B. Preferential use of LAIV among young children was not supported.
Keywords: influenza vaccine; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

Comment in
-
Editorial Commentary: Influenza Vaccine Effectiveness: A Glass Both Half Full and Half Empty.Clin Infect Dis. 2016 Dec 15;63(12):1574-1576. doi: 10.1093/cid/ciw637. Epub 2016 Oct 4. Clin Infect Dis. 2016. PMID: 27702767 No abstract available.
References
-
- Centers for Disease Control and Prevention. Characteristics associated with seasonal influenza vaccination of preschool children—Oregon, 2006–2008. Morb Mortal Wkly Rep (MMWR) 2011; 60:981–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

