Making sense out of spinal cord somatosensory development
- PMID: 27702783
- PMCID: PMC5087618
- DOI: 10.1242/dev.139592
Making sense out of spinal cord somatosensory development
Abstract
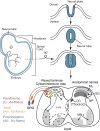
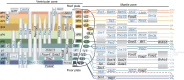
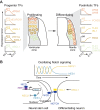
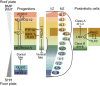
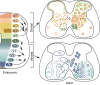
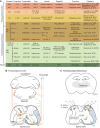
The spinal cord integrates and relays somatosensory input, leading to complex motor responses. Research over the past couple of decades has identified transcription factor networks that function during development to define and instruct the generation of diverse neuronal populations within the spinal cord. A number of studies have now started to connect these developmentally defined populations with their roles in somatosensory circuits. Here, we review our current understanding of how neuronal diversity in the dorsal spinal cord is generated and we discuss the logic underlying how these neurons form the basis of somatosensory circuits.
Keywords: Cutaneous; Dorsal spinal cord development; Itch; Mechanosensation; Neuroepithelium; Nociception; Pain; Proprioception; Pruriception; Thermosensation; Touch; Transcription factor networks; Vertebrate neural tube.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons.Development. 2005 Jun;132(12):2709-19. doi: 10.1242/dev.01859. Epub 2005 May 18. Development. 2005. PMID: 15901662 Free PMC article.
-
Spinal Circuits for Touch, Pain, and Itch.Annu Rev Physiol. 2018 Feb 10;80:189-217. doi: 10.1146/annurev-physiol-022516-034303. Epub 2017 Sep 27. Annu Rev Physiol. 2018. PMID: 28961064 Free PMC article. Review.
-
Prrxl1 is required for the generation of a subset of nociceptive glutamatergic superficial spinal dorsal horn neurons.Dev Dyn. 2010 Jun;239(6):1684-94. doi: 10.1002/dvdy.22305. Dev Dyn. 2010. PMID: 20503365
-
Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches.Front Neural Circuits. 2020 Jun 12;14:31. doi: 10.3389/fncir.2020.00031. eCollection 2020. Front Neural Circuits. 2020. PMID: 32595458 Free PMC article. Review.
-
Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord.Development. 2004 Nov;131(21):5393-403. doi: 10.1242/dev.01379. Epub 2004 Oct 6. Development. 2004. PMID: 15469980
Cited by
-
The Role of Neurodevelopmental Pathways in Brain Tumors.Front Cell Dev Biol. 2021 Apr 27;9:659055. doi: 10.3389/fcell.2021.659055. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34012965 Free PMC article. Review.
-
Spinal dI4 Interneuron Differentiation From Human Pluripotent Stem Cells.Front Mol Neurosci. 2022 Apr 8;15:845875. doi: 10.3389/fnmol.2022.845875. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35465095 Free PMC article.
-
In vitro atlas of dorsal spinal interneurons reveals Wnt signaling as a critical regulator of progenitor expansion.Cell Rep. 2022 Jul 19;40(3):111119. doi: 10.1016/j.celrep.2022.111119. Cell Rep. 2022. PMID: 35858555 Free PMC article.
-
Altered Sensory Neuron Development in CMT2D Mice Is Site-Specific and Linked to Increased GlyRS Levels.Front Cell Neurosci. 2020 Aug 11;14:232. doi: 10.3389/fncel.2020.00232. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848623 Free PMC article.
-
An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception.Diversity (Basel). 2021 Aug;13(8):364. doi: 10.3390/d13080364. Epub 2021 Aug 7. Diversity (Basel). 2021. PMID: 35505776 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

