Randomized phase 2 trial of ixazomib and dexamethasone in relapsed multiple myeloma not refractory to bortezomib
- PMID: 27702799
- PMCID: PMC5114487
- DOI: 10.1182/blood-2016-05-717769
Randomized phase 2 trial of ixazomib and dexamethasone in relapsed multiple myeloma not refractory to bortezomib
Abstract
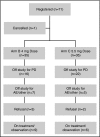
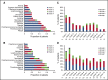
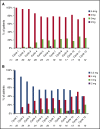
Proteasome inhibitors have become an integral part of myeloma therapy. Considerable efforts have gone into optimizing this therapeutic approach to obtain maximal proteasome inhibition with least toxicity. Ixazomib is the first oral proteasome inhibitor to enter the clinic and has been studied as a single agent as well as in various combinations. The current trial was designed to examine the efficacy and toxicity of combining 2 different doses of ixazomib (4 mg and 5.5 mg given weekly for 3 of 4 weeks) with 40 mg weekly of dexamethasone, in relapsed myeloma. Seventy patients were enrolled, 35 patients randomly assigned to each ixazomib dose. Overall, 30 (43%; 95% confidence interval, 31-55) of the patients achieved a confirmed partial response or better, with 31% achieving a response with 4 mg and 54% with 5.5 mg of ixazomib. The median event-free survival (EFS) for the entire study population was 8.4 months; 1-year overall survival was 96%. The EFS was 5.7 months for patients with prior bortezomib exposure and 11.0 months for bortezomib-naïve patients. A grade 3 or 4 adverse event considered at least possibly related to treatment was seen in 11 (32%) patients at 4 mg and in 21 (60%) at 5.5 mg. Dose reductions were more frequent with 5.5 mg dose. Overall, the ixazomib with dexamethasone has good efficacy in relapsed myeloma, is well-tolerated and with higher response rate at 5.5 mg, albeit with more toxicity. This study was registered at www.clinicaltrials.gov as #NCT01415882.
© 2016 by The American Society of Hematology.
Figures


References
-
- Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7(1):9-16. - PubMed
-
- Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. . Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20(22):4420-4427. - PubMed
-
- Richardson PG, Sonneveld P, Schuster M, et al. . Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557-3560. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

