Label-free imaging of the native, living cellular nanoarchitecture using partial-wave spectroscopic microscopy
- PMID: 27702891
- PMCID: PMC5081614
- DOI: 10.1073/pnas.1608198113
Label-free imaging of the native, living cellular nanoarchitecture using partial-wave spectroscopic microscopy
Abstract
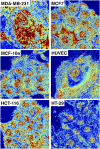
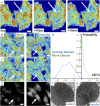
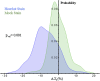
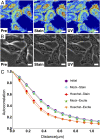
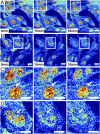
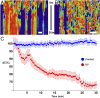
The organization of chromatin is a regulator of molecular processes including transcription, replication, and DNA repair. The structures within chromatin that regulate these processes span from the nucleosomal (10-nm) to the chromosomal (>200-nm) levels, with little known about the dynamics of chromatin structure between these scales due to a lack of quantitative imaging technique in live cells. Previous work using partial-wave spectroscopic (PWS) microscopy, a quantitative imaging technique with sensitivity to macromolecular organization between 20 and 200 nm, has shown that transformation of chromatin at these length scales is a fundamental event during carcinogenesis. As the dynamics of chromatin likely play a critical regulatory role in cellular function, it is critical to develop live-cell imaging techniques that can probe the real-time temporal behavior of the chromatin nanoarchitecture. Therefore, we developed a live-cell PWS technique that allows high-throughput, label-free study of the causal relationship between nanoscale organization and molecular function in real time. In this work, we use live-cell PWS to study the change in chromatin structure due to DNA damage and expand on the link between metabolic function and the structure of higher-order chromatin. In particular, we studied the temporal changes to chromatin during UV light exposure, show that live-cell DNA-binding dyes induce damage to chromatin within seconds, and demonstrate a direct link between higher-order chromatin structure and mitochondrial membrane potential. Because biological function is tightly paired with structure, live-cell PWS is a powerful tool to study the nanoscale structure-function relationship in live cells.
Keywords: DNA damage; cell dynamics; chromatin; microscopy; mitochondrial metabolism.
Conflict of interest statement
V.B., H.S., and H.K.R. are cofounders of Nanocytomics, LLC.
Figures
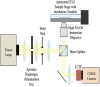

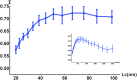
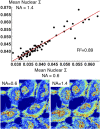
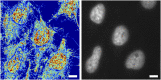


References
-
- Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

