Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer's disease
- PMID: 27702899
- PMCID: PMC5081632
- DOI: 10.1073/pnas.1611073113
Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer's disease
Abstract
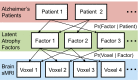
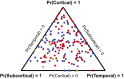
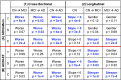
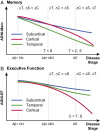
We used a data-driven Bayesian model to automatically identify distinct latent factors of overlapping atrophy patterns from voxelwise structural MRIs of late-onset Alzheimer's disease (AD) dementia patients. Our approach estimated the extent to which multiple distinct atrophy patterns were expressed within each participant rather than assuming that each participant expressed a single atrophy factor. The model revealed a temporal atrophy factor (medial temporal cortex, hippocampus, and amygdala), a subcortical atrophy factor (striatum, thalamus, and cerebellum), and a cortical atrophy factor (frontal, parietal, lateral temporal, and lateral occipital cortices). To explore the influence of each factor in early AD, atrophy factor compositions were inferred in beta-amyloid-positive (Aβ+) mild cognitively impaired (MCI) and cognitively normal (CN) participants. All three factors were associated with memory decline across the entire clinical spectrum, whereas the cortical factor was associated with executive function decline in Aβ+ MCI participants and AD dementia patients. Direct comparison between factors revealed that the temporal factor showed the strongest association with memory, whereas the cortical factor showed the strongest association with executive function. The subcortical factor was associated with the slowest decline for both memory and executive function compared with temporal and cortical factors. These results suggest that distinct patterns of atrophy influence decline across different cognitive domains. Quantification of this heterogeneity may enable the computation of individual-level predictions relevant for disease monitoring and customized therapies. Factor compositions of participants and code used in this article are publicly available for future research.
Keywords: Alzheimer’s disease heterogeneity; Alzheimer’s disease subtypes; mental disorder subtypes; unsupervised machine learning; voxel-based morphometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
- K25 EB013649/EB/NIBIB NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- S10 RR019307/RR/NCRR NIH HHS/United States
- F32 AG044054/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- R21 AG050122/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R41 AG052246/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- K01 AG051718/AG/NIA NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

