LasR Variant Cystic Fibrosis Isolates Reveal an Adaptable Quorum-Sensing Hierarchy in Pseudomonas aeruginosa
- PMID: 27703072
- PMCID: PMC5050340
- DOI: 10.1128/mBio.01513-16
LasR Variant Cystic Fibrosis Isolates Reveal an Adaptable Quorum-Sensing Hierarchy in Pseudomonas aeruginosa
Abstract
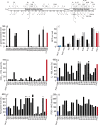
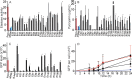
Chronic Pseudomonas aeruginosa infections cause significant morbidity in patients with cystic fibrosis (CF). Over years to decades, P. aeruginosa adapts genetically as it establishes chronic lung infections. Nonsynonymous mutations in lasR, the quorum-sensing (QS) master regulator, are common in CF. In laboratory strains of P. aeruginosa, LasR activates transcription of dozens of genes, including that for another QS regulator, RhlR. Despite the frequency with which lasR coding variants have been reported to occur in P. aeruginosa CF isolates, little is known about their consequences for QS. We sequenced lasR from 2,583 P. aeruginosa CF isolates. The lasR sequences of 580 isolates (22%) coded for polypeptides that differed from the conserved LasR polypeptides of well-studied laboratory strains. This collection included 173 unique lasR coding variants, 116 of which were either missense or nonsense mutations. We studied 31 of these variants. About one-sixth of the variant LasR proteins were functional, including 3 with nonsense mutations, and in some LasR-null isolates, genes that are LasR dependent in laboratory strains were nonetheless expressed. Furthermore, about half of the LasR-null isolates retained RhlR activity. Therefore, in some CF isolates the QS hierarchy is altered such that RhlR quorum sensing is independent of LasR regulation. Our analysis challenges the view that QS-silent P. aeruginosa is selected during the course of a chronic CF lung infection. Rather, some lasR sequence variants retain functionality, and many employ an alternate QS strategy involving RhlR.
Importance: Chronic Pseudomonas aeruginosa infections, such as those in patients with the genetic disease cystic fibrosis, are notable in that mutants with defects in the quorum-sensing transcription factor LasR frequently arise. In laboratory strains of P. aeruginosa, quorum sensing activates transcription of dozens of genes, many of which encode virulence factors, such as secreted proteases and hydrogen cyanide synthases. In well-studied laboratory strains, LasR-null mutants have a quorum-sensing-deficient phenotype. Therefore, the presence of LasR variants in chronic infections has been interpreted to indicate that quorum-sensing-regulated products are not important for those infections. We report that some P. aeruginosa LasR variant clinical isolates are not LasR-null mutants, and others have uncoupled a second quorum-sensing system, the RhlR system, from LasR regulation. In these uncoupled isolates, RhlR independently activates at least some quorum-sensing-dependent genes. Our findings suggest that quorum sensing plays a role in chronic P. aeruginosa infections, despite the emergence of LasR coding variants.
Copyright © 2016 Feltner et al.
Figures
References
-
- Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi:10.1073/pnas.0602138103. - DOI - PMC - PubMed
-
- D’Argenio DA, Wu M, Hoffman LR, Kulasekara HD, Déziel E, Smith EE, Nguyen H, Ernst RK, Larson Freeman TJ, Spencer DH, Brittnacher M, Hayden HS, Selgrade S, Klausen M, Goodlett DR, Burns JL, Ramsey BW, Miller SI. 2007. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol 64:512–533. doi:10.1111/j.1365-2958.2007.05678.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
