Biobank attributes associated with higher patient participation: a randomized study
- PMID: 27703145
- PMCID: PMC5159767
- DOI: 10.1038/ejhg.2016.132
Biobank attributes associated with higher patient participation: a randomized study
Abstract
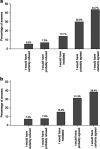
The objectives of the study were to assess patients' intent to participate in a hospital-based biobank and to explore the factors associated with higher participation. A 23-item questionnaire was developed to survey a random sample of patients in a Swiss university hospital. Two vignettes describing hypothetical biobanks were incorporated in the survey and patients were asked whether they would agree to participate. Three factors were randomly manipulated in each vignette using a factorial design: cancer-oriented research vs general consent, one vs several reviews of the patient's chart, and genetic vs blood protein analyses (first vignette); blood sample vs oral swabbing, local vs international project, and a follow-up visit vs no visit (second vignette). Of the 1140 respondents, 73.6 and 69.6%, respectively, agreed to participate in the biobank. Biospecimen collection via oral swabbing, single chart review, and no follow-up were associated with higher participation. Participation was also higher among younger patients, Europeans, patients who had a positive opinion on research, and blood/organ donors. Biobanking was supported by a majority of patients, especially if biospecimens were collected through non-invasive techniques or if data collection was done once. The scope of consent, the scale of the project, or the tests performed on biospecimens did not influence participation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Public's attitudes on participation in a biobank for research: an Italian survey.BMC Med Ethics. 2014 Nov 26;15:81. doi: 10.1186/1472-6939-15-81. BMC Med Ethics. 2014. PMID: 25425352 Free PMC article.
-
Patients' Knowledge and Attitude Toward Biobanks in Eastern Morocco.Biopreserv Biobank. 2020 Jun;18(3):189-195. doi: 10.1089/bio.2019.0047. Epub 2020 Mar 24. Biopreserv Biobank. 2020. PMID: 32207985
-
Minority Participation in Biobanks: An Essential Key to Progress.Methods Mol Biol. 2019;1897:43-50. doi: 10.1007/978-1-4939-8935-5_5. Methods Mol Biol. 2019. PMID: 30539433 Review.
-
Assessment of knowledge about biobanking among healthcare students and their willingness to donate biospecimens.BMC Med Ethics. 2017 May 2;18(1):32. doi: 10.1186/s12910-017-0195-8. BMC Med Ethics. 2017. PMID: 28464877 Free PMC article.
-
A template for broad consent in biobank research. Results and explanation of an evidence and consensus-based development process.Eur J Med Genet. 2016 Jun;59(6-7):295-309. doi: 10.1016/j.ejmg.2016.04.002. Epub 2016 Apr 26. Eur J Med Genet. 2016. PMID: 27130428 Review.
Cited by
-
Study design factors influencing patients' willingness to participate in clinical research: a randomised vignette-based study.BMC Med Res Methodol. 2020 Apr 26;20(1):93. doi: 10.1186/s12874-020-00979-z. BMC Med Res Methodol. 2020. PMID: 32336266 Free PMC article. Clinical Trial.
-
PIRATE project: point-of-care, informatics-based randomised controlled trial for decreasing overuse of antibiotic therapy in Gram-negative bacteraemia.BMJ Open. 2017 Jul 13;7(7):e017996. doi: 10.1136/bmjopen-2017-017996. BMJ Open. 2017. PMID: 28710229 Free PMC article. Clinical Trial.
-
Community recommendations on biobank governance: Results from a deliberative community engagement in California.PLoS One. 2017 Feb 24;12(2):e0172582. doi: 10.1371/journal.pone.0172582. eCollection 2017. PLoS One. 2017. PMID: 28235046 Free PMC article.
-
Public preferences towards data management and governance in Swiss biobanks: results from a nationwide survey.BMJ Open. 2022 Aug 26;12(8):e060844. doi: 10.1136/bmjopen-2022-060844. BMJ Open. 2022. PMID: 36028266 Free PMC article.
-
Knowledge and perceptions of South African blood donors towards biobanking and stool donation.S Afr J Infect Dis. 2024 Oct 31;39(1):645. doi: 10.4102/sajid.v39i1.645. eCollection 2024. S Afr J Infect Dis. 2024. PMID: 39507519 Free PMC article.
References
-
- Khoury MJ, Millikan R, Little J, Gwinn M: The emergence of epidemiology in the genomics age. Int J Epidemiol 2004; 33: 936–944. - PubMed
-
- Ashburn TT, Wilson SK, Eisenstein BI: Human tissue research in the genomic era of medicine: balancing individual and societal interests. Arch Intern Med 2000; 160: 3377–3384. - PubMed
-
- Hofmann B: Broadening consent—-and diluting ethics? J Med Ethics 2009; 35: 125–129. - PubMed
-
- Mooser V, Currat C: The Lausanne Institutional Biobank: a new resource to catalyse research in personalised medicine and pharmaceutical sciences. Swiss Med Wkly 2014; 144: w14033. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources