The origin of efficient triplet state population in sulfur-substituted nucleobases
- PMID: 27703148
- PMCID: PMC5059480
- DOI: 10.1038/ncomms13077
The origin of efficient triplet state population in sulfur-substituted nucleobases
Abstract
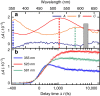
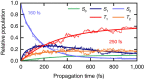
Elucidating the photophysical mechanisms in sulfur-substituted nucleobases (thiobases) is essential for designing prospective drugs for photo- and chemotherapeutic applications. Although it has long been established that the phototherapeutic activity of thiobases is intimately linked to efficient intersystem crossing into reactive triplet states, the molecular factors underlying this efficiency are poorly understood. Herein we combine femtosecond transient absorption experiments with quantum chemistry and nonadiabatic dynamics simulations to investigate 2-thiocytosine as a necessary step to unravel the electronic and structural elements that lead to ultrafast and near-unity triplet-state population in thiobases in general. We show that different parts of the potential energy surfaces are stabilized to different extents via thionation, quenching the intrinsic photostability of canonical DNA and RNA nucleobases. These findings satisfactorily explain why thiobases exhibit the fastest intersystem crossing lifetimes measured to date among bio-organic molecules and have near-unity triplet yields, whereas the triplet yields of canonical nucleobases are nearly zero.
Figures




Similar articles
-
On the decay of the triplet state of thionucleobases.Phys Chem Chem Phys. 2017 May 24;19(20):12674-12682. doi: 10.1039/c7cp02050c. Phys Chem Chem Phys. 2017. PMID: 28474025
-
2,4-Dithiothymine as a potent UVA chemotherapeutic agent.J Am Chem Soc. 2014 Dec 31;136(52):17930-3. doi: 10.1021/ja510611j. Epub 2014 Dec 17. J Am Chem Soc. 2014. PMID: 25506742
-
Excited State Lifetimes of Sulfur-Substituted DNA and RNA Monomers Probed Using the Femtosecond Fluorescence Up-Conversion Technique.Molecules. 2020 Jan 29;25(3):584. doi: 10.3390/molecules25030584. Molecules. 2020. PMID: 32013184 Free PMC article.
-
Photochemical and Photodynamical Properties of Sulfur-Substituted Nucleic Acid Bases.Photochem Photobiol. 2019 Jan;95(1):33-58. doi: 10.1111/php.12975. Epub 2018 Sep 9. Photochem Photobiol. 2019. PMID: 29978490 Review.
-
Photochemistry of nucleic acid bases and their thio- and aza-analogues in solution.Top Curr Chem. 2015;355:245-327. doi: 10.1007/128_2014_554. Top Curr Chem. 2015. PMID: 25238718 Review.
Cited by
-
Single-atom replacement as a general approach towards visible-light/near-infrared heavy-atom-free photosensitizers for photodynamic therapy.Chem Sci. 2020 Jun 2;11(26):6701-6708. doi: 10.1039/d0sc02286a. eCollection 2020 Jul 14. Chem Sci. 2020. PMID: 32953031 Free PMC article.
-
Radiation Induced One-Electron Oxidation of 2-Thiouracil in Aqueous Solutions.Molecules. 2019 Dec 2;24(23):4402. doi: 10.3390/molecules24234402. Molecules. 2019. PMID: 31810289 Free PMC article.
-
Photochemical and thermochemical pathways to S2 and polysulfur formation in the atmosphere of Venus.Nat Commun. 2022 Jul 30;13(1):4425. doi: 10.1038/s41467-022-32170-x. Nat Commun. 2022. PMID: 35907911 Free PMC article.
-
Conditionally Activatable Pyroptosis-Inducing Agents for Cancer Therapy.Small Sci. 2023 Dec 15;4(2):2300135. doi: 10.1002/smsc.202300135. eCollection 2024 Feb. Small Sci. 2023. PMID: 40212358 Free PMC article.
-
Tautomer aspects in the excited-state dynamics in 2-thiocytosine: intersystem crossing in the absence of the thiocarbonyl group.Chem Sci. 2025 Jul 23;16(33):15015-15028. doi: 10.1039/d5sc01442e. eCollection 2025 Aug 20. Chem Sci. 2025. PMID: 40708875 Free PMC article.
References
-
- Carell T. et al. Structure and function of noncanonical nucleobases. Angew Chem. Int. Ed. Engl. 51, 7110–7131 (2012). - PubMed
-
- Carbon J., David H. & Studier M. H. Thiobases in Escherchia coli transfer RNA: 2-thiocytosine and 5-methylaminomethyl-2-thiouracil. Science 161, 1146–1147 (1968). - PubMed
-
- Wojciechowski F. & Leumann C. J. Alternative DNA base-pairs: from efforts to expand the genetic code to potential material applications. Chem. Soc. Rev. 40, 5669–5679 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

