Elastic Scattering Spectroscopy (ESS): an Instrument-Concept for Dynamics of Complex (Bio-) Systems From Elastic Neutron Scattering
- PMID: 27703184
- PMCID: PMC5050422
- DOI: 10.1038/srep34266
Elastic Scattering Spectroscopy (ESS): an Instrument-Concept for Dynamics of Complex (Bio-) Systems From Elastic Neutron Scattering
Abstract
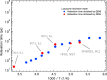
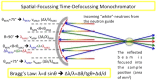
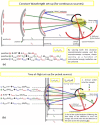
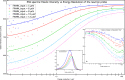
A new type of neutron-scattering spectroscopy is presented that is designed specifically to measure dynamics in bio-systems that are difficult to obtain in any other way. The temporal information is largely model-free and is analogous to relaxation processes measured with dielectric spectroscopy, but provides additional spacial and geometric aspects of the underlying dynamics. Numerical simulations of the basic instrument design show the neutron beam can be highly focussed, giving efficiency gains that enable the use of small samples. Although we concentrate on continuous neutron sources, the extension to pulsed neutron sources is proposed, both requiring minimal data-treatment and being broadly analogous with dielectric spectroscopy, they will open the study of dynamics to new areas of biophysics.
Figures
References
-
- Doster W., Cusack S. & Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature 337, 754–756 (1989). - PubMed
-
- Rasmussen B. F., Stock A. M., Ringe D. & Petsko G. A. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature 357, 423 (1992). - PubMed
-
- Lim M., Jackson T. A. & Anfinrud P. A. Ultrafast rotation and trapping of carbon monoxide dissociated from myoglobin. Nat. Struct. Biol. 4, 209–214 (1997). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

