Epigraph: A Vaccine Design Tool Applied to an HIV Therapeutic Vaccine and a Pan-Filovirus Vaccine
- PMID: 27703185
- PMCID: PMC5050445
- DOI: 10.1038/srep33987
Epigraph: A Vaccine Design Tool Applied to an HIV Therapeutic Vaccine and a Pan-Filovirus Vaccine
Abstract
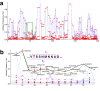
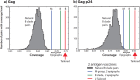
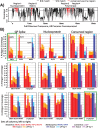
Epigraph is an efficient graph-based algorithm for designing vaccine antigens to optimize potential T-cell epitope (PTE) coverage. Epigraph vaccine antigens are functionally similar to Mosaic vaccines, which have demonstrated effectiveness in preliminary HIV non-human primate studies. In contrast to the Mosaic algorithm, Epigraph is substantially faster, and in restricted cases, provides a mathematically optimal solution. Epigraph furthermore has new features that enable enhanced vaccine design flexibility. These features include the ability to exclude rare epitopes from a design, to optimize population coverage based on inexact epitope matches, and to apply the code to both aligned and unaligned input sequences. Epigraph was developed to provide practical design solutions for two outstanding vaccine problems. The first of these is a personalized approach to a therapeutic T-cell HIV vaccine that would provide antigens with an excellent match to an individual's infecting strain, intended to contain or clear a chronic infection. The second is a pan-filovirus vaccine, with the potential to protect against all known viruses in the Filoviradae family, including ebolaviruses. A web-based interface to run the Epigraph tool suite is available (http://www.hiv.lanl.gov/content/sequence/EPIGRAPH/epigraph.html).
Conflict of interest statement
K.F., L.J.P., B.K. and J.T. are co-inventors on a patent application (PCT/US15/54067) on “HIV Vaccines Comprising One or More Population Episensus Antigens.” Authors LJP and KF have a significant financial interest in TomegaVax Inc., a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

