The antenna of horse stomach bot flies: morphology and phylogenetic implications (Oestridae, Gasterophilinae: Gasterophilus Leach)
- PMID: 27703229
- PMCID: PMC5050557
- DOI: 10.1038/srep34409
The antenna of horse stomach bot flies: morphology and phylogenetic implications (Oestridae, Gasterophilinae: Gasterophilus Leach)
Abstract
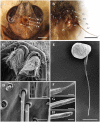
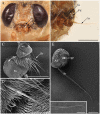
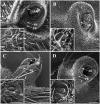
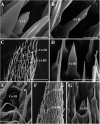
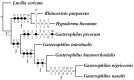
Antennae are among the most elaborate sensory organs in adult flies, and they provide rich information for phylogenic studies. The antennae of five out of eight species of Gasterophilus Leach (G. haemorrhoidalis (Linnaeus), G. intestinalis (De Geer), G. nasalis (Linnaeus), G. nigricornis (Loew) and G. pecorum (Fabricius)), were examined using scanning electron microscopy. The general morphology, including distribution, type, size, and ultrastructure of antennal sensilla were presented, and the definition of auriculate sensilla and sensory pits were updated and clarified. Eighteen antennal characters were selected to construct the first species-level phylogeny of this genus. The monophyly of Gasterophilus was supported by the presence of coeloconic sensilla III on the antennal arista. The species-level cladogram showed G. pecorum branching off at the base, and the remaining species forming the topology (G. intestinalis+ (G. haemorrhoidalis+ (G. nasalis+ G. nigricornis))). Our research shows the importance of the antennal ultrastructure as a reliable source for phylogenetic analysis.
Figures







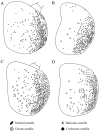


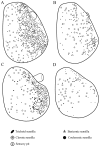
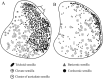

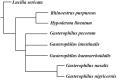

Similar articles
-
Sensilla on the antennal funiculus of the horse stomach bot fly, Gasterophilus nigricornis.Med Vet Entomol. 2012 Sep;26(3):314-22. doi: 10.1111/j.1365-2915.2011.01007.x. Epub 2012 Jan 26. Med Vet Entomol. 2012. PMID: 22276803
-
Cuticular structures on antennae of the bot fly, Portschinskia magnifica (Diptera: Oestridae).Parasitol Res. 2012 Oct;111(4):1651-9. doi: 10.1007/s00436-012-3004-9. Epub 2012 Jul 10. Parasitol Res. 2012. PMID: 22777702
-
Molecular and morphological characterization of third instar Palaearctic horse stomach bot fly larvae (Oestridae: Gasterophilinae, Gasterophilus).Vet Parasitol. 2018 Oct 15;262:56-74. doi: 10.1016/j.vetpar.2018.09.011. Epub 2018 Sep 24. Vet Parasitol. 2018. PMID: 30389013
-
Comparative morphology of antennal ultrastructure in Tachinidae parasitoid flies (Diptera): The phylogenetic importance of antennal sensilla.Arthropod Struct Dev. 2022 Nov;71:101202. doi: 10.1016/j.asd.2022.101202. Epub 2022 Aug 26. Arthropod Struct Dev. 2022. PMID: 36037740
-
Ultrastructural investigation of antennae in three cutaneous myiasis flies: Melophagus ovinus, Hippobosca equina, and Hippobosca longipennis (Diptera: Hippoboscidae).Parasitol Res. 2015 May;114(5):1887-96. doi: 10.1007/s00436-015-4376-4. Epub 2015 Feb 25. Parasitol Res. 2015. PMID: 25707367
Cited by
-
Sensillar Ultrastructure of the Antennae and Maxillary Palps of the Warble Fly Oestromyia leporina (Pallas, 1778) (Diptera: Oestridae).Insects. 2024 Jul 28;15(8):574. doi: 10.3390/insects15080574. Insects. 2024. PMID: 39194779 Free PMC article.
-
Morphological and Ultrastructural Characterization of Antennal Sensilla and the Detection of Floral Scent Volatiles in Eupeodes corollae (Diptera: Syrphidae).Front Neuroanat. 2021 Dec 16;15:791900. doi: 10.3389/fnana.2021.791900. eCollection 2021. Front Neuroanat. 2021. PMID: 34975421 Free PMC article.
-
Antennal and palpal sensilla of three predatory Lispe species (Diptera: Muscidae): an ultrastructural investigation.Sci Rep. 2021 Sep 15;11(1):18357. doi: 10.1038/s41598-021-97677-7. Sci Rep. 2021. PMID: 34526584 Free PMC article.
-
Insect Antennal Morphology: The Evolution of Diverse Solutions to Odorant Perception.Yale J Biol Med. 2018 Dec 21;91(4):457-469. eCollection 2018 Dec. Yale J Biol Med. 2018. PMID: 30588211 Free PMC article. Review.
-
Morphology and Distribution of Antennal Sensilla in an Egg Parasitoid Wasp, Anastatus disparis (Hymenoptera: Eupelmidae).J Insect Sci. 2022 Nov 1;22(6):6. doi: 10.1093/jisesa/ieac072. J Insect Sci. 2022. PMID: 36469364 Free PMC article.
References
-
- Giroux M., Pape T. & Wheeler T. A. Towards a phylogeny of the flesh flies (Diptera: Sarcophagidae): morphology and phylogenetic implications of the acrophallus in the subfamily Sarcophaginae. Zool J Linn Soc-Lond 158, 740–778 (2010).
-
- Lambkin C. L. et al.. The phylogenetic relationships among infraorders and superfamilies of Diptera based on morphological evidence. Syst Entomol 38, 164–179 (2012).
-
- Yeates D. K. & Wiegmann B. M. Phylogeny and evolution of Diptera: recent insights and new perspectives. In: The evolutionary biology of flies (eds Yeates D. K. & Wiegmann B. M.) 14–44 (Columbia University Press, 2005).
-
- Araujo D. P., Tuan M. J. M., Yew J. Y. & Meier R. Analysing small insect glands with UV-LDI MS: high-resolution spatial analysis reveals the chemical composition and use of the osmeterium secretion in Themira superba (Sepsidae: Diptera). J Evolution Biol 27, 1744–1750 (2014). - PubMed
-
- Wipfler B., Courtney G. W., Craig D. A. & Beutel R. G. First μ-CT-based 3D reconstruction of a dipteran larva—the head morphology of Protanyderus (Tanyderidae) and its phylogenetic implications. J Morphol 273, 968–980 (2012). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

