Reverse-topology membrane scission by the ESCRT proteins
- PMID: 27703243
- PMCID: PMC5198518
- DOI: 10.1038/nrm.2016.121
Reverse-topology membrane scission by the ESCRT proteins
Abstract
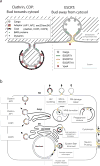
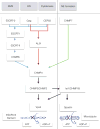
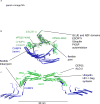
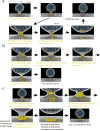
The narrow membrane necks formed during viral, exosomal and intra-endosomal budding from membranes, as well as during cytokinesis and related processes, have interiors that are contiguous with the cytosol. Severing these necks involves action from the opposite face of the membrane as occurs during the well-characterized formation of coated vesicles. This 'reverse' (or 'inverse')-topology membrane scission is carried out by the endosomal sorting complex required for transport (ESCRT) proteins, which form filaments, flat spirals, tubes and conical funnels that are thought to direct membrane remodelling and scission. Their assembly, and their disassembly by the ATPase vacuolar protein sorting-associated 4 (VPS4) have been intensively studied, but the mechanism of scission has been elusive. New insights from cryo-electron microscopy and various types of spectroscopy may finally be close to rectifying this situation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

