Gender Differences in Adipocyte Metabolism and Liver Cancer Progression
- PMID: 27703473
- PMCID: PMC5029146
- DOI: 10.3389/fgene.2016.00168
Gender Differences in Adipocyte Metabolism and Liver Cancer Progression
Abstract
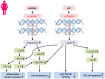
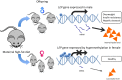
Liver cancer is the third most common cancer type and the second leading cause of deaths in men. Large population studies have demonstrated remarkable gender disparities in the incidence and the cumulative risk of liver cancer. A number of emerging risk factors regarding metabolic alterations associated with obesity, diabetes and dyslipidemia have been ascribed to the progression of non-alcoholic fatty liver diseases (NAFLD) and ultimately liver cancer. The deregulation of fat metabolism derived from excessive insulin, glucose, and lipid promotes cancer-causing inflammatory signaling and oxidative stress, which eventually triggers the uncontrolled hepatocellular proliferation. This review presents the current standing on the gender differences in body fat compositions and their mechanistic linkage with the development of NAFLD-related liver cancer, with an emphasis on genetic, epigenetic and microRNA control. The potential roles of sex hormones in instructing adipocyte metabolic programs may help unravel the mechanisms underlying gender dimorphism in liver cancer and identify the metabolic targets for disease management.
Keywords: adipocyte; adipokine; adiponectin; epigenetic; gender dimorphism; hepatocellular carcinoma; leptin; metabolism.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

