Changes in Insulin Resistance After Initiation of Raltegravir or Protease Inhibitors With Tenofovir-Emtricitabine: AIDS Clinical Trials Group A5260s
- PMID: 27704026
- PMCID: PMC5047417
- DOI: 10.1093/ofid/ofw174
Changes in Insulin Resistance After Initiation of Raltegravir or Protease Inhibitors With Tenofovir-Emtricitabine: AIDS Clinical Trials Group A5260s
Abstract
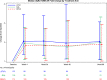
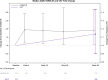
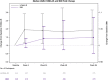
Background. Antiretroviral therapy (ART) can alter glucose metabolism, but little data exist on the association of raltegravir (RAL) with insulin resistance. Methods. A5260s was a substudy of A5257, a prospective open-label randomized trial in which human immunodeficiency virus (HIV)-infected treatment-naive participants were randomized to tenofovir-emtricitabine (TDF/FTC) plus atazanavir-ritonavir (ATV/r), darunavir-ritonavir (DRV/r), or RAL over 96 weeks. Baseline and changes in insulin resistance as estimated by the homeostatic model assessment of insulin resistance (HOMA-IR) were assessed. Wilcoxon rank-sum tests were used to assess shifts in the distribution of fold increase from baseline between treatment arms, and Spearman correlation was used to assess associations between HOMA-IR and measures of inflammation and body composition. Results. Three hundred twenty-eight participants were randomized; 90% were male, baseline median age was 36, HIV ribonucleic acid copies were 4.55 log10 copies/mL, and CD4 cell count was 349/mm3. Overall, HOMA-IR increased significantly after 4 weeks (1.9-fold change; 95% confidence interval, 1.73-2.05) then plateaued over the remainder of the study. Changes in HOMA-IR were not different between the arms (P ≥ .23). Changes in HOMA-IR were associated with changes in body mass index at weeks 48 and 96 (r = 0.12-0.22; P ≤ .04). There was a trend with increases in HOMA-IR and increases in visceral abdominal fat at week 96 (r = 0.12; P = .06). At 48 and 96 weeks, HOMA-IR correlated with interleukin-6, high-sensitivity C-reactive protein, and soluble CD163 (r = 0.16-0.27; P ≤ .003). Conclusions. Insulin resistance increased rapidly and then plateaued in treatment-naive participants initiating ART with TDF/FTC, and no differences were found with RAL when compared with ATV/r or DRV/r.
Keywords: inflammatory markers; insulin resistance; raltegravir.
Figures
References
-
- Capeau J, Bouteloup V, Katlama C et al. . Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012; 26:303–14. - PubMed
-
- Brown TT, Cole SR, Li X et al. . Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165:1179–84. - PubMed
-
- Brown TT, Li X, Cole SR et al. . Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS 2005; 19:1375–83. - PubMed
Grants and funding
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- K24 AI056933/AI/NIAID NIH HHS/United States
- K08 AI108272/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- K23 HD088295/HD/NICHD NIH HHS/United States
- R01 HL095126/HL/NHLBI NIH HHS/United States
- R01 HL095132/HL/NHLBI NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

