Not spreading in reverse: The dewetting of a liquid film into a single drop
- PMID: 27704042
- PMCID: PMC5040479
- DOI: 10.1126/sciadv.1600183
Not spreading in reverse: The dewetting of a liquid film into a single drop
Abstract
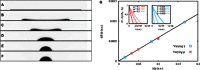
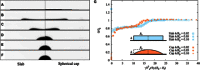
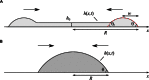
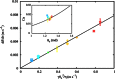
Wetting and dewetting are both fundamental modes of motion of liquids on solid surfaces. They are critically important for processes in biology, chemistry, and engineering, such as drying, coating, and lubrication. However, recent progress in wetting, which has led to new fields such as superhydrophobicity and liquid marbles, has not been matched by dewetting. A significant problem has been the inability to study the model system of a uniform film dewetting from a nonwetting surface to a single macroscopic droplet-a barrier that does not exist for the reverse wetting process of a droplet spreading into a film. We report the dewetting of a dielectrophoresis-induced film into a single equilibrium droplet. The emergent picture of the full dewetting dynamics is of an initial regime, where a liquid rim recedes at constant speed and constant dynamic contact angle, followed by a relatively short exponential relaxation of a spherical cap shape. This sharply contrasts with the reverse wetting process, where a spreading droplet follows a smooth sequence of spherical cap shapes. Complementary numerical simulations and a hydrodynamic model reveal a local dewetting mechanism driven by the equilibrium contact angle, where contact line slip dominates the dewetting dynamics. Our conclusions can be used to understand a wide variety of processes involving liquid dewetting, such as drop rebound, condensation, and evaporation. In overcoming the barrier to studying single film-to-droplet dewetting, our results provide new approaches to fluid manipulation and uses of dewetting, such as inducing films of prescribed initial shapes and slip-controlled liquid retraction.
Keywords: Dewetting; contact-line dynamics; dielectrowetting; slip.
Figures




References
-
- P.-G. de Gennes, F. Brochard-Wyart, D. Quere, Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves (Springer Science & Business Media, 2013).
-
- Bonn D., Eggers J., Indekeu J., Meunier J., Rolley E., Wetting and spreading. Rev. Mod. Phys. 81, 739–805 (2009).
-
- Sharma A., Ruckenstein E., Dewetting of solids by the formation of holes in macroscopic liquid films. J. Colloid Interface Sci. 133, 358–368 (1989).
-
- Gentili D., Foschi G., Valle F., Cavallini M., Biscarini F., Applications of dewetting in micro and nanotechnology. Chem. Soc. Rev. 41, 4430–4443 (2012). - PubMed
-
- Tian D., Song Y., Jiang L., Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 42, 5184–5209 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

