Human multipotent adult progenitor cells enhance islet function and revascularisation when co-transplanted as a composite pellet in a mouse model of diabetes
- PMID: 27704164
- PMCID: PMC6518081
- DOI: 10.1007/s00125-016-4120-3
Human multipotent adult progenitor cells enhance islet function and revascularisation when co-transplanted as a composite pellet in a mouse model of diabetes
Abstract
Aims/hypothesis: Hypoxia in the initial days after islet transplantation leads to considerable loss of islet mass and contributes to disappointing outcomes in the clinical setting. The aim of the present study was to investigate whether co-transplantation of human non-endothelial bone marrow-derived multipotent adult progenitor cells (MAPCs), which are non-immunogenic and can secrete angiogenic growth factors during the initial days after implantation, could improve islet engraftment and survival.
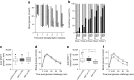
Methods: Islets (150) were co-transplanted, with or without human MAPCs (2.5 × 105) as separate or composite pellets, under the kidney capsule of syngeneic alloxan-induced diabetic C57BL/6 mice. Blood glucose levels were frequently monitored and IPGTTs were carried out. Grafts and serum were harvested at 2 and 5 weeks after transplantation to assess outcome.
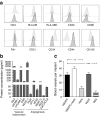
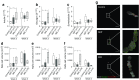
Results: Human MAPCs produced high amounts of angiogenic growth factors, including vascular endothelial growth factor, in vitro and in vivo, as demonstrated by the induction of neo-angiogenesis in the chorioallantoic membrane assay. Islet-human MAPC co-transplantation as a composite pellet significantly improved the outcome of islet transplantation as measured by the initial glycaemic control, diabetes reversal rate, glucose tolerance and serum C-peptide concentration compared with the outcome following transplantation of islets alone. Histologically, a higher blood vessel area and density in addition to a higher vessel/islet ratio were detected in recipients of islet-human MAPC composites.
Conclusions/interpretation: The present data suggest that co-transplantation of mouse pancreatic islets with human MAPCs, which secrete high amounts of angiogenic growth factors, enhance islet graft revascularisation and subsequently improve islet graft function.
Keywords: Islet; Revascularisation; Stem cells; Type 1 diabetes.
Conflict of interest statement
Duality of interest
PS and BV are compensated employees of ReGenesys BVBA and have compensated stock options from Athersys, Inc. All others declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
All authors were involved in the acquisition, analysis or interpretation of data. All authors were involved in the study concept and design and drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
CM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Figures

References
-
- Campbell MD, Walker M, Bracken RM, et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015;3:e000085. doi: 10.1136/bmjdrc-2015-000085. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

