Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance)
- PMID: 27704226
- PMCID: PMC5189982
- DOI: 10.1007/s10549-016-4006-6
Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance)
Abstract
Objective: It had been previously shown that patients who receive neoadjuvant systemic therapy (NST) are more likely to undergo breast-conserving therapy (BCT) than those who have primary surgery. However, the frequency with which patients who are not BCT-eligible prior to NST convert to BCT-eligible with treatment is unknown. To document this conversion rate in a subset of patients expected to have a high clinical response rate to NST, we studied surgical assessment and management of patients enrolled on a randomized neoadjuvant trial for stage II-III HER2-positive breast cancer (HER2 + BC)(CALGB 40601).
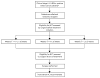
Methods: The treating surgeon assessed BCT candidacy based on clinico-radiographic criteria both before and after NST. Definitive breast surgical management was at surgeon and patient discretion. We sought to determine (1) the conversion rate from BCT-ineligible to BCT-eligible (2) the percentage of BCT-eligible patients who chose breast conservation, and (3) the rate of successful BCT. We also evaluated surgeon-determined factors for BCT-ineligibility and the correlation between BCT eligibility and pathologic complete response (pCR).
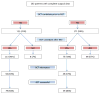
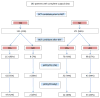
Results: Of 292 patients with pre- and post-NST surgical assessments, 59 % were non-BCT candidates at baseline. Of the 43 % of these patients who converted with NST, 67 % opted for BCT, with an 80 % success rate. NST increased the BCT-eligible rate from 41 to 64 %. Common factors cited for BCT-ineligibility prior to NST including tumor size (56 %) and probable poor cosmetic outcome (26 %) were reduced by 67 and 75 %, respectively, with treatment, while multicentricity, the second most common factor (33 %), fell by only 16 %. Since 23 % of the BCT-eligible patients chose mastectomy, BCT was the final surgical procedure in just 40 % of the patients. Patients considered BCT-eligible both at baseline and after NST had a pCR rate of 55 %, while patients who were BCT-ineligible prior to NST had the same pCR rate (44 %) whether they converted to BCT-eligible or not.
Conclusions: Many patients with HER2 + BC deemed ineligible for BCT at baseline can be converted to BCT-eligible with NST; excluding patients with multicentric disease substantially increases that percentage. In converted patients who opt for BCT, the success rate is similar to that of patients considered BCT-eligible at baseline. Whether a BCT-ineligible patient converts to BCT eligibility or not does not appear to affect the likelihood of achieving a pCR. Despite the efficacy of NST in this patient cohort, only 40 % of patients had successful BCT; further research into why BCT-eligible patients often opt for mastectomy is needed.
Keywords: Breast conserving therapy; HER2-positive breast cancer; Neoadjuvant therapy.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures
References
-
- Barry PA, Schiavon G. Primary systemic treatment in the management of operable breast cancer: best surgical approach for diagnosis, biological evaluation, and research. J Natl Cancer Inst Monogr. 2015;2015(51):4–8. - PubMed
-
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(5):778–785. - PubMed
-
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(22):4224–4237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous