Early implant-associated osteomyelitis results in a peri-implanted bacterial reservoir
- PMID: 27704604
- PMCID: PMC5298028
- DOI: 10.1111/apm.12597
Early implant-associated osteomyelitis results in a peri-implanted bacterial reservoir
Abstract
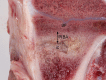
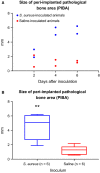
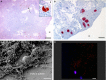
Implant-associated osteomyelitis (IAO) is a common complication in orthopedic surgery. The aim of this study was to elucidate how deep IAO can go into the peri-implanted bone tissue within a week. The study was performed in a porcine model of IAO. A small steel implant and either 104 CFU/kg body weight of Staphylococcus aureus or saline was inserted into the right tibial bone of 12 pigs. The animals were consecutively killed on day 2, 4 and 6 following implantation. Bone tissue around the implant was histologically evaluated. Identification of S. aureus was performed immunohistochemically on tissue section and with scanning electron microscopy and peptide nucleic acid in situ hybridization on implants. The distance of the peri-implanted pathological bone area (PIBA), measured perpendicular to the implant, was significantly larger in infected animals compared to controls (p = 0.0014). The largest differences were seen after 4 and 6 days of inoculation, where PIBA measurements of up to 6 mm were observed. Positive S. aureus bacteria were identified on implants and from 25 μm to 6 mm into PIBA. This is important knowledge for optimizing outcomes of surgical debridement in osteomyelitis.
Keywords: S. aureus; Implant-associated osteomyelitis; animal experiment; histopathology.
© 2016 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Medical Microbiology and Pathology.
Figures

References
-
- Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997;336:999–1007. - PubMed
-
- Sendi P, Zimmerli W. Antimicrobial treatment concepts for orthopedic device‐related infections. Clin Microbiol Infect 2012;18:1176–84. - PubMed
-
- Krenek L, Farng E, Zingmond D, SooHoo NF. Complication and revision rates following total elbow arthroplasty. J Hand Surg Am 2011;36:68–73. - PubMed
-
- Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopedic biofilm infections. FEMS Immunol Med Microbiol 2012;65:158–68. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

