Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor
- PMID: 27704638
- PMCID: PMC8502039
- DOI: 10.1002/jbmr.3011
Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor
Abstract
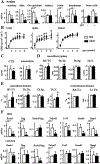
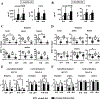
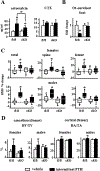
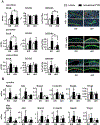
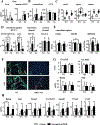
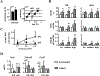
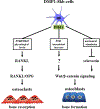
Osteocytes integrate the responses of bone to mechanical and hormonal stimuli by poorly understood mechanisms. We report here that mice with conditional deletion of the parathyroid hormone (PTH) receptor 1 (Pth1r) in dentin matrix protein 1 (DMP1)-8kb-expressing cells (cKO) exhibit a modest decrease in bone resorption leading to a mild increase in cancellous bone without changes in cortical bone. However, bone resorption in response to endogenous chronic elevation of PTH in growing or adult cKO mice induced by a low calcium diet remained intact, because the increased bone remodeling and bone loss was indistinguishable from that exhibited by control littermates. In contrast, the bone gain and increased bone formation in cancellous and cortical bone induced by daily injections of PTH and the periosteal bone apposition induced by axial ulna loading were markedly reduced in cKO mice compared to controls. Remarkably, however, wild-type (WT) control littermates and transgenic mice overexpressing SOST injected daily with PTH exhibit similar activation of Wnt/β-catenin signaling, increased bone formation, and cancellous and cortical bone gain. Taken together, these findings demonstrate that Pth1r in DMP1-8kb-expressing cells is required to maintain basal levels of bone resorption but is dispensable for the catabolic action of chronic PTH elevation; and it is essential for the anabolic actions of daily PTH injections and mechanical loading. However, downregulation of Sost/sclerostin, previously shown to be required for bone anabolism induced by mechanical loading, is not required for PTH-induced bone gain, showing that other mechanisms downstream of the Pth1r in DMP1-8kb-expressing cells are responsible for the hormonal effect. © 2016 American Society for Bone and Mineral Research.
Keywords: GENETIC ANIMAL MODELS; MOLECULAR PATHWAYS-REMODELING; OSTEOCYTES; PTH/VITD/FGF23; WNT/Β-CATENIN/LRPS.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosures
All authors state that they have no conflicts of interest.
Figures
References
-
- Saliba W, El-Haddad B. Secondary hyperparathyroidism: pathophysiology and treatment. J Am Board Fam Med. 2009;22:574–81. - PubMed
-
- Ma YL, Cain RL, Halladay DL, et al. Catabolic effects of continuous human pth (1–38) in vivo is associated with sustained stimulation of rankl and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

