Synthesis of 13(R)-Hydroxy-7Z,10Z,13R,14E,16Z,19Z Docosapentaenoic Acid (13R-HDPA) and Its Biosynthetic Conversion to the 13-Series Resolvins
- PMID: 27704804
- PMCID: PMC5149404
- DOI: 10.1021/acs.jnatprod.6b00634
Synthesis of 13(R)-Hydroxy-7Z,10Z,13R,14E,16Z,19Z Docosapentaenoic Acid (13R-HDPA) and Its Biosynthetic Conversion to the 13-Series Resolvins
Abstract
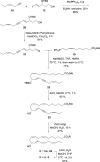
Specialized pro-resolving lipid mediators are biosynthesized during the resolution phase of acute inflammation from n-3 polyunsaturated fatty acids. Recently, the isolation and identification of the four novel mediators denoted 13-series resolvins, namely, RvT1 (1), RvT2 (2), RvT3 (3) and RvT4 (4), were reported, which showed potent bioactions characteristic for specialized pro-resolving lipid mediators. Herein, based on results from LC/MS-MS metabololipidomics and the stereoselective synthesis of 13(R)-hydroxy-7Z,10Z,13R,14E,16Z,19Z docosapentaenoic acid (13R-HDPA, 5), we provide direct evidence that the four novel mediators 1-4 are all biosynthesized from the pivotal intermediate 5. The UV and LC/MS-MS results from synthetic 13R-HDPA (5) matched those from endogenously and biosynthetically produced material obtained from in vivo infectious exudates, endothelial cells, and human recombinant COX-2 enzyme. Stereochemically pure 5 was obtained with the use of a chiral pool starting material that installed the configuration at the C-13 atom as R. Two stereoselective Z-Wittig reactions and two Z-selective reductions of internal alkynes afforded the geometrically pure alkene moieties in 5. Incubation of 5 with isolated human neutrophils gave all four RvTs. The results presented herein provide new knowledge on the biosynthetic pathways and the enzymatic origin of RvTs 1-4.
Conflict of interest statement
Notes J. D. and C. N. S. have filed patents on RvT1 (1), RvT2 (2), RvT3 (3), RvT4 (4), 13R-HDPA (5) and related compounds. C. N. S.’s interests are reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict of interest policies.
Figures









Similar articles
-
Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages.Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):12232-12237. doi: 10.1073/pnas.1607003113. Epub 2016 Oct 6. Proc Natl Acad Sci U S A. 2016. PMID: 27791009 Free PMC article.
-
Total synthesis of the lipid mediator PD1n-3 DPA: configurational assignments and anti-inflammatory and pro-resolving actions.J Nat Prod. 2014 Apr 25;77(4):910-6. doi: 10.1021/np4009865. Epub 2014 Feb 27. J Nat Prod. 2014. PMID: 24576195 Free PMC article.
-
Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation.Clin Chem. 2012 Oct;58(10):1476-84. doi: 10.1373/clinchem.2012.190199. Epub 2012 Aug 21. Clin Chem. 2012. PMID: 22912397
-
Recent advances in the chemistry and biology of anti-inflammatory and specialized pro-resolving mediators biosynthesized from n-3 docosapentaenoic acid.Bioorg Med Chem Lett. 2017 Jun 1;27(11):2259-2266. doi: 10.1016/j.bmcl.2017.03.079. Epub 2017 Mar 31. Bioorg Med Chem Lett. 2017. PMID: 28408222 Review.
-
Biology and Total Synthesis of n-3 Docosapentaenoic Acid-Derived Specialized Pro-Resolving Mediators.Molecules. 2024 Jun 14;29(12):2833. doi: 10.3390/molecules29122833. Molecules. 2024. PMID: 38930898 Free PMC article. Review.
Cited by
-
Base-Induced Sulfoxide-Sulfenate Rearrangement of 2-Sulfinyl Dienes for the Regio- and Stereoselective Synthesis of Enantioenriched Dienyl Diols.J Org Chem. 2023 Mar 17;88(6):3697-3713. doi: 10.1021/acs.joc.2c02931. Epub 2023 Mar 3. J Org Chem. 2023. PMID: 36868575 Free PMC article.
-
Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology.Br J Pharmacol. 2019 Apr;176(8):1024-1037. doi: 10.1111/bph.14336. Epub 2018 Jun 3. Br J Pharmacol. 2019. PMID: 29679485 Free PMC article. Review.
-
Specialized pro-resolving mediator network: an update on production and actions.Essays Biochem. 2020 Sep 23;64(3):443-462. doi: 10.1042/EBC20200018. Essays Biochem. 2020. PMID: 32885825 Free PMC article. Review.
-
Some Biogenetic Considerations Regarding the Marine Natural Product (-)-Mucosin.Molecules. 2019 Nov 15;24(22):4147. doi: 10.3390/molecules24224147. Molecules. 2019. PMID: 31731797 Free PMC article.
-
Roles of Resolvins in Chronic Inflammatory Response.Int J Mol Sci. 2022 Nov 28;23(23):14883. doi: 10.3390/ijms232314883. Int J Mol Sci. 2022. PMID: 36499209 Free PMC article. Review.
References
-
- Dalli J.; Chiang N.; Serhan C. N. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, E4753–E4761. 10.1073/pnas.1415006111. - DOI - PMC - PubMed
- Dalli J.; Ramon S.; Norris P. C.; Colas R. A.; Serhan C. N. FASEB J. 2015, 29, 2120–2136. 10.1096/fj.14-268441. - DOI - PMC - PubMed
- Ramon S.; Dalli J.; Sanger J. M.; Winkler J. W.; Aursnes M.; Tungen J. E.; Hansen T. V.; Serhan C. N. Am. J. Pathol. 2016, 186, 962–973. 10.1016/j.ajpath.2015.12.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

