Highly Efficient Cascade Reaction for Selective Formation of Spirocyclobutenes from Dienallenes via Palladium-Catalyzed Oxidative Double Carbocyclization-Carbonylation-Alkynylation
- PMID: 27704805
- PMCID: PMC5084063
- DOI: 10.1021/jacs.6b09240
Highly Efficient Cascade Reaction for Selective Formation of Spirocyclobutenes from Dienallenes via Palladium-Catalyzed Oxidative Double Carbocyclization-Carbonylation-Alkynylation
Abstract
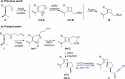
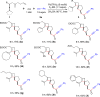
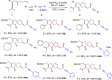
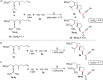
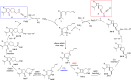
A highly selective cascade reaction that allows the direct transformation of dienallenes to spirocyclobutenes (spiro[3.4]octenes) as single diastereoisomers has been developed. The reaction involves formation of overall four C-C bonds and proceeds via a palladium-catalyzed oxidative transformation with insertion of olefin, olefin, and carbon monoxide. Under slightly different reaction conditions, an additional CO insertion takes place to give spiro[4.4]nonenes with formation of overall five C-C bonds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
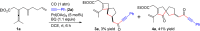
Similar articles
-
Highly Selective Cascade C-C Bond Formation via Palladium- Catalyzed Oxidative Carbonylation-Carbocyclization-Carbonylation-Alkynylation of Enallenes.J Am Chem Soc. 2015 Sep 23;137(37):11868-71. doi: 10.1021/jacs.5b06828. Epub 2015 Sep 15. J Am Chem Soc. 2015. PMID: 26356201
-
Palladium-Catalyzed Oxidative Cascade Carbonylative Spirolactonization of Enallenols.Angew Chem Int Ed Engl. 2017 Mar 13;56(12):3221-3225. doi: 10.1002/anie.201612384. Epub 2017 Feb 17. Angew Chem Int Ed Engl. 2017. PMID: 28211212
-
Control of Selectivity in Palladium(II)-Catalyzed Oxidative Transformations of Allenes.Acc Chem Res. 2018 Jun 19;51(6):1520-1531. doi: 10.1021/acs.accounts.8b00138. Epub 2018 May 24. Acc Chem Res. 2018. PMID: 29792667
-
Palladium-catalyzed oxidative domino carbocyclization-carbonylation-alkynylation of enallenes.Org Lett. 2014 Aug 15;16(16):4174-7. doi: 10.1021/ol501862z. Epub 2014 Aug 4. Org Lett. 2014. PMID: 25090018
-
Palladium-Catalyzed Cascade Cyclization/Alkynylation Reactions.Chem Asian J. 2019 Dec 2;14(23):4114-4128. doi: 10.1002/asia.201901202. Epub 2019 Nov 4. Chem Asian J. 2019. PMID: 31637860 Review.
Cited by
-
Palladium-Catalyzed Carbonylations: Application in Complex Natural Product Total Synthesis and Recent Developments.J Org Chem. 2023 Apr 21;88(8):4925-4941. doi: 10.1021/acs.joc.2c02746. Epub 2023 Jan 27. J Org Chem. 2023. PMID: 36705327 Free PMC article. Review.
-
Amino-Supported Palladium Catalyst for Chemo- and Stereoselective Domino Reactions.Angew Chem Int Ed Engl. 2021 Jan 11;60(2):670-674. doi: 10.1002/anie.202011708. Epub 2020 Nov 10. Angew Chem Int Ed Engl. 2021. PMID: 32969105 Free PMC article.
-
Sequential Insertion of Alkynes, Alkenes, and CO into the Pd-C Bond of ortho-Palladated Primary Phenethylamines: from η3-Allyl Complexes and Enlarged Palladacycles to Functionalized Arylalkylamines.Organometallics. 2021 Feb 22;40(4):539-556. doi: 10.1021/acs.organomet.0c00787. Epub 2021 Jan 29. Organometallics. 2021. PMID: 35264820 Free PMC article.
-
Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines.Molecules. 2017 Feb 22;22(2):328. doi: 10.3390/molecules22020328. Molecules. 2017. PMID: 28241452 Free PMC article.
-
Electrochemical Palladium-Catalyzed Oxidative Carbonylation-Cyclization of Enallenols.Angew Chem Int Ed Engl. 2022 Dec 5;61(49):e202212131. doi: 10.1002/anie.202212131. Epub 2022 Nov 4. Angew Chem Int Ed Engl. 2022. PMID: 36222322 Free PMC article.
References
-
-
For selected reviews, see:
- Rios R. Chem. Soc. Rev. 2012, 41, 1060.10.1039/C1CS15156H. - DOI - PubMed
- Kotha S.; Deb A.; Lahiri K.; Manivannan E. Synthesis 2009, 2009, 165.10.1055/s-0028-1083300. - DOI
- Ding K.; Han Z.; Wang Z. Chem. - Asian J. 2009, 4, 32.10.1002/asia.200800192. - DOI - PubMed
- D'yakonov V. A.; Trapeznikova O. A.; de Meijere A.; Dzhemilev U. M. Chem. Rev. 2014, 114, 5775.10.1021/cr400291c. - DOI - PubMed
-
-
-
For selected reviews involving construction of a quaternary carbon center, see:
- Steven A.; Overman L. E. Angew. Chem., Int. Ed. 2007, 46, 5488.10.1002/anie.200700612. - DOI - PubMed
- Shimizu M. Angew. Chem., Int. Ed. 2011, 50, 5998.10.1002/anie.201101720. - DOI - PubMed
- Wang B.; Tu Y. Acc. Chem. Res. 2011, 44, 1207.10.1021/ar200082p. - DOI - PubMed
-
-
-
For selected examples, see:
- Zheng Y.; Tice C. M.; Singh S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673.10.1016/j.bmcl.2014.06.081. - DOI - PubMed
- Welsch M. E.; Snyder S. A.; Stockwell B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.10.1016/j.cbpa.2010.02.018. - DOI - PMC - PubMed
- Kotha S.; Mandal K. Tetrahedron Lett. 2004, 45, 1391.10.1016/j.tetlet.2003.12.075. - DOI
- Li F.; Tartakoff S. S.; Castle S. L. J. Am. Chem. Soc. 2009, 131, 6674.10.1021/ja9024403. - DOI - PubMed
- Chiang Y.-M.; Kuo Y.-H. Tetrahedron Lett. 2003, 44, 5125.10.1016/S0040-4039(03)01116-X. - DOI
-
-
- Xie J.; Zhou Q. Acc. Chem. Res. 2008, 41, 581.10.1021/ar700137z. - DOI - PubMed
- Zhu S.; Cai Y.; Mao H.; Xie J.; Zhou Q. Nat. Chem. 2010, 2, 546.10.1038/nchem.651. - DOI - PubMed
- Coulter M. M.; Kou K. G. M.; Galligan B.; Dong V. M. J. Am. Chem. Soc. 2010, 132, 16330.10.1021/ja107198e. - DOI - PubMed
- Zhu S.; Song X.; Li Y.; Cai Y.; Zhou Q. J. Am. Chem. Soc. 2010, 132, 16374.10.1021/ja1078464. - DOI - PubMed
-
-
For selected reviews, see:
- Fürstner A. Chem. Soc. Rev. 2009, 38, 3208.10.1039/b816696j. - DOI - PubMed
- Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696.10.1021/ar400239v. - DOI - PubMed
- Cohen D. T.; Scheidt K. A. Chem. Sci. 2012, 3, 53.10.1039/C1SC00621E. - DOI - PMC - PubMed
- Grossmann A.; Enders D. Angew. Chem., Int. Ed. 2012, 51, 314.10.1002/anie.201105415. - DOI - PubMed
- Fensterbank L.; Malacria M. Acc. Chem. Res. 2014, 47, 953.10.1021/ar4002334. - DOI - PubMed
- Hopkinson M. N.; Richter C.; Schedler M.; Glorius F. Nature 2014, 510, 485.10.1038/nature13384. - DOI - PubMed
-
For selected examples, see:
- Dugal-Tessier J.; O'Bryan E. A.; Schroeder T. B. H.; Cohen D. T.; Scheidt K. A. Angew. Chem., Int. Ed. 2012, 51, 4963.10.1002/anie.201201643. - DOI - PMC - PubMed
- Li J.; Sahoo B.; Daniliuc C. G.; Glorius F. Angew. Chem., Int. Ed. 2014, 53, 10515.10.1002/anie.201405178. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

