Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT)
- PMID: 27705888
- PMCID: PMC5837371
- DOI: 10.1093/eurheartj/ehw387
Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT)
Erratum in
-
Corrigendum.Eur Heart J. 2018 Mar 21;39(12):998. doi: 10.1093/eurheartj/ehw625. Eur Heart J. 2018. PMID: 28025195 Free PMC article. No abstract available.
Abstract
Background: Selective cyclooxygenase-2 inhibitors and conventional non-selective non-steroidal anti-inflammatory drugs (nsNSAIDs) have been associated with adverse cardiovascular (CV) effects. We compared the CV safety of switching to celecoxib vs. continuing nsNSAID therapy in a European setting.
Method: Patients aged 60 years and over with osteoarthritis or rheumatoid arthritis, free from established CV disease and taking chronic prescribed nsNSAIDs, were randomized to switch to celecoxib or to continue their previous nsNSAID. The primary endpoint was hospitalization for non-fatal myocardial infarction or other biomarker positive acute coronary syndrome, non-fatal stroke or CV death analysed using a Cox model with a pre-specified non-inferiority limit of 1.4 for the hazard ratio (HR).
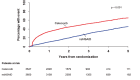
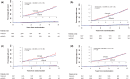
Results: In total, 7297 participants were randomized. During a median 3-year follow-up, fewer subjects than expected developed an on-treatment (OT) primary CV event and the rate was similar for celecoxib, 0.95 per 100 patient-years, and nsNSAIDs, 0.86 per 100 patient-years (HR = 1.12, 95% confidence interval, 0.81-1.55; P = 0.50). Comparable intention-to-treat (ITT) rates were 1.14 per 100 patient-years with celecoxib and 1.10 per 100 patient-years with nsNSAIDs (HR = 1.04; 95% confidence interval, 0.81-1.33; P = 0.75). Pre-specified non-inferiority was achieved in the ITT analysis. The upper bound of the 95% confidence limit for the absolute increase in OT risk associated with celecoxib treatment was two primary events per 1000 patient-years exposure. There were only 15 adjudicated secondary upper gastrointestinal complication endpoints (0.078/100 patient-years on celecoxib vs. 0.053 on nsNSAIDs OT, 0.078 vs. 0.053 ITT). More gastrointestinal serious adverse reactions and haematological adverse reactions were reported on nsNSAIDs than celecoxib, but more patients withdrew from celecoxib than nsNSAIDs (50.9% patients vs. 30.2%; P < 0.0001).
Interpretation: In subjects 60 years and over, free from CV disease and taking prescribed chronic nsNSAIDs, CV events were infrequent and similar on celecoxib and nsNSAIDs. There was no advantage of a strategy of switching prescribed nsNSAIDs to prescribed celecoxib. This study excluded an increased risk of the primary endpoint of more than two events per 1000 patient-years associated with switching to prescribed celecoxib.
Clinical trial registration: https://clinicaltrials.gov/show/NCT00447759; Unique identifier: NCT00447759.
Keywords: Arthritis; Cardiovascular; Celecoxib; NSAIDs.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology
Figures

Comment in
-
Traditional NSAIDs and coxibs: is one better than the other?Eur Heart J. 2017 Jun 14;38(23):1851-1852. doi: 10.1093/eurheartj/ehw507. Eur Heart J. 2017. PMID: 28329068 No abstract available.
References
-
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Matchaba P, Gimona A, Hawkey CJ; TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364: 665–74. - PubMed
-
- Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C.. Coxib and traditional NSAID Trialists' (CNT) Collaboration, Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. - PMC - PubMed
-
- Mukherjee D, Nissen SE, Topol EJ.. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954–9. - PubMed
-
- http://www.fda.gov/Drugs/DrugSafety/ucm451800.htm (10 July 2015).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

