Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment
- PMID: 27705910
- PMCID: PMC5340181
- DOI: 10.18632/oncotarget.12249
Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment
Abstract
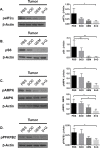
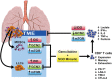
Indoleamine 2,3-dioxygenase (IDO) has been implicated in immune evasion by tumors. Upregulation of this tryptophan (Trp)-catabolizing enzyme, in tumor cells and myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment (TME), leads to Trp depletion that impairs cytotoxic T cell responses and survival; however, exact mechanisms remain incompletely understood. We previously reported that a combination therapy of gemcitabine and a superoxide dismutase mimetic promotes anti-tumor immunity in a mouse model of lung cancer by inhibiting MDSCs, enhancing polyfunctional response of CD8+ memory T cells, and extending survival. Here, we show that combination therapy targets IDO signaling, specifically in MDSCs, tumor cells, and CD8+ T cells infiltrating the TME. Deficiency of IDO caused significant reduction in tumor burden, tumor-infiltrating MDSCs, GM-CSF, MDSC survival and infiltration of programmed death receptor-1 (PD-1)-expressing CD8+ T cells compared to controls. IDO-/- MDSCs downregulated nutrient-sensing AMP-activated protein kinase (AMPK) activity, but IDO-/- CD8+ T cells showed AMPK activation associated with enhanced effector function. Our studies provide proof-of-concept for the efficacy of this combination therapy in inhibiting IDO and T cell exhaustion in a syngeneic model of lung cancer and provide mechanistic insights for IDO-dependent metabolic reprogramming of MDSCs that reduces T cell exhaustion and regulates anti-tumor immunity.
Keywords: combination therapy; lung cancer; metabolism; myeloid-derived suppressor cells; indoleamine 2,3-dioxygenase.
Conflict of interest statement
There are no financial conflicts of interest for any of the authors listed.
Figures





References
-
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer research. 2012;72:5435–5440. - PubMed
-
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clinica chimica acta. 2006;364:82–90. - PubMed
-
- Kurz K, Schroecksnadel S, Weiss G, Fuchs D. Association between increased tryptophan degradation and depression in cancer patients. Current opinion in clinical nutrition and metabolic care. 2011;14:49–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

