RND3 promotes Snail 1 protein degradation and inhibits glioblastoma cell migration and invasion
- PMID: 27705942
- PMCID: PMC5347701
- DOI: 10.18632/oncotarget.12396
RND3 promotes Snail 1 protein degradation and inhibits glioblastoma cell migration and invasion
Abstract
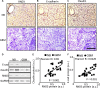
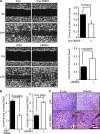
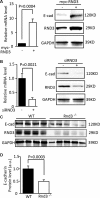
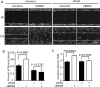
Activation of Snail1 signaling promotes the migration and invasion of multiple tumors, including glioblastoma multiforme (GBM). However, the molecular mechanism that augments Snail1 signaling during GBM cell migration and invasion remains largely unknown. Identification of the factors that regulate Snail1 signaling is critical to block tumor cell migration and invasion. By screening human GBM specimens, we found that the expression levels of small GTPase RND3 positively correlated with the expression levels of E-cadherin and claudin, the glioblastoma migration biomarkers negatively regulated by Snail1. Downregulation of E-cadherin and claudin has been associated with the migration and invasion of GBM cells. We demonstrated that RND3 functioned as an endogenous inhibitor of the Snail-directed transcriptional regulation. RND3 physically interacted with Snail1 protein, enhanced Snail1 ubiquitination, and facilitated the protein degradation. Forced expression of RND3 inhibited Snail1 activity, which in turn blocked glioblastoma cell migration and invasion in vitro in cell culture and in vivo in GBM xenograft mice. In contrast, downregulation of RND3 augmented Snail1 activity, and subsequently decreased E-cadherin expression, eventually promoted glioblastoma cell migration and invasion. The pro-migration induced by RND3 downregulation was attenuated by Snail1 knockdown. The findings partially explain why Snail1 activity is augmented in GBM, and defines a new function of RND3 in GBM cell migration and invasion.
Keywords: RND3; multiform glioblastoma; snail1 signaling.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Ferrari-Amorotti G, Fragliasso V, Esteki R, Prudente Z, Soliera AR, Cattelani S, Manzotti G, Grisendi G, Dominici M, Pieraccioli M, Raschella G, Chiodoni C, Colombo MP, et al. Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion. Cancer research. 2013;73:235–245. - PMC - PubMed
-
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. - PubMed
-
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

