Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells
- PMID: 27706128
- PMCID: PMC5380589
- DOI: 10.1038/mi.2016.83
Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells
Abstract
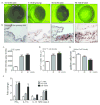
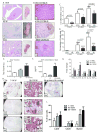
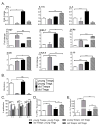
In both humans and animal models, the development of Sjögren syndrome (SS) and non-SS keratoconjunctivitis sicca (KCS) increases with age. Here, we investigated the ocular surface and lacrimal gland (LG) phenotype of NOD.B10.H2b mice at 7-14, 45-50, and 96-100 weeks. Aged mice develop increased corneal permeability, CD4+ T-cell infiltration, and conjunctival goblet cell loss. Aged mice have LG atrophy with increased lymphocyte infiltration and inflammatory cytokine levels. An increase in the frequency of CD4+Foxp3+ T regulatory cells (Tregs) was observed with age in the cervical lymph node (CLN), spleen, and LG. These CD4+CD25+ cells lose suppressive ability, while maintaining expression of Foxp3 (forkhead box P3) and producing interleukin-17 (IL-17) and interferon-γ (IFN-γ). An increase of Foxp3+IL-17+ or Foxp3+IFN-γ+ cells was observed in the LG and LG-draining CLN. In adoptive transfer experiments, recipients of either purified Tregs or purified T effector cells from aged donors developed lacrimal keratoconjunctivitis, whereas recipients of young Tregs or young T effector cells failed to develop disease. Overall, these results suggest inflammatory cytokine-producing CD4+Foxp3+ cells participate in the pathogenesis of age-related ocular surface disease.
Figures
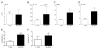

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

