Large Variations in HIV-1 Viral Load Explained by Shifting-Mosaic Metapopulation Dynamics
- PMID: 27706164
- PMCID: PMC5051940
- DOI: 10.1371/journal.pbio.1002567
Large Variations in HIV-1 Viral Load Explained by Shifting-Mosaic Metapopulation Dynamics
Abstract
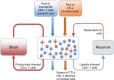
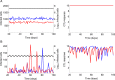
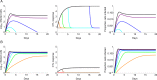
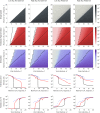
The viral population of HIV-1, like many pathogens that cause systemic infection, is structured and differentiated within the body. The dynamics of cellular immune trafficking through the blood and within compartments of the body has also received wide attention. Despite these advances, mathematical models, which are widely used to interpret and predict viral and immune dynamics in infection, typically treat the infected host as a well-mixed homogeneous environment. Here, we present mathematical, analytical, and computational results that demonstrate that consideration of the spatial structure of the viral population within the host radically alters predictions of previous models. We study the dynamics of virus replication and cytotoxic T lymphocytes (CTLs) within a metapopulation of spatially segregated patches, representing T cell areas connected by circulating blood and lymph. The dynamics of the system depend critically on the interaction between CTLs and infected cells at the within-patch level. We show that for a wide range of parameters, the system admits an unexpected outcome called the shifting-mosaic steady state. In this state, the whole body's viral population is stable over time, but the equilibrium results from an underlying, highly dynamic process of local infection and clearance within T-cell centers. Notably, and in contrast to previous models, this new model can explain the large differences in set-point viral load (SPVL) observed between patients and their distribution, as well as the relatively low proportion of cells infected at any one time, and alters the predicted determinants of viral load variation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Notwithstanding Circumstantial Alibis, Cytotoxic T Cells Can Be Major Killers of HIV-1-Infected Cells.J Virol. 2016 Jul 27;90(16):7066-7083. doi: 10.1128/JVI.00306-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27226367 Free PMC article.
-
Accumulation of Pol Mutations Selected by HLA-B*52:01-C*12:02 Protective Haplotype-Restricted Cytotoxic T Lymphocytes Causes Low Plasma Viral Load Due to Low Viral Fitness of Mutant Viruses.J Virol. 2017 Jan 31;91(4):e02082-16. doi: 10.1128/JVI.02082-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27903797 Free PMC article.
-
An HIV epidemic model based on viral load dynamics: value in assessing empirical trends in HIV virulence and community viral load.PLoS Comput Biol. 2014 Jun 19;10(6):e1003673. doi: 10.1371/journal.pcbi.1003673. eCollection 2014 Jun. PLoS Comput Biol. 2014. PMID: 24945322 Free PMC article.
-
What do mathematical models tell us about killing rates during HIV-1 infection?Immunol Lett. 2015 Nov;168(1):1-6. doi: 10.1016/j.imlet.2015.07.009. Epub 2015 Aug 13. Immunol Lett. 2015. PMID: 26279491 Review.
-
Correlates of cytotoxic T-lymphocyte-mediated virus control: implications for immunosuppressive infections and their treatment.Philos Trans R Soc Lond B Biol Sci. 2000 Aug 29;355(1400):1059-70. doi: 10.1098/rstb.2000.0643. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11186307 Free PMC article. Review.
Cited by
-
The effective rate of influenza reassortment is limited during human infection.PLoS Pathog. 2017 Feb 7;13(2):e1006203. doi: 10.1371/journal.ppat.1006203. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28170438 Free PMC article.
-
Relational concurrency, stages of infection, and the evolution of HIV set point viral load.Virus Evol. 2018 Nov 21;4(2):vey032. doi: 10.1093/ve/vey032. eCollection 2018 Jul. Virus Evol. 2018. PMID: 30483403 Free PMC article.
-
Link between the numbers of particles and variants founding new HIV-1 infections depends on the timing of transmission.Virus Evol. 2019 Jan 30;5(1):vey038. doi: 10.1093/ve/vey038. eCollection 2019 Jan. Virus Evol. 2019. PMID: 30723550 Free PMC article.
-
Estimating hepatitis B virus cccDNA persistence in chronic infection.Virus Evol. 2020 Aug 25;7(1):veaa063. doi: 10.1093/ve/veaa063. eCollection 2021 Jan. Virus Evol. 2020. PMID: 33732502 Free PMC article.
-
Sexual role and HIV-1 set point viral load among men who have sex with men.Epidemics. 2019 Mar;26:68-76. doi: 10.1016/j.epidem.2018.08.006. Epub 2018 Aug 30. Epidemics. 2019. PMID: 30193771 Free PMC article.
References
-
- Bormann FH, Likens GE. Pattern and process in a forested ecosystem: disturbance, development and the steady state based on the Hubbard Brook ecosystem NY Springer. New York: Springer-Verlag; 1979.
-
- de Wolf F, Spijkerman I, Schellekens PT, Langendam M, Kuiken C, Bakker M, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11: 1799–1806. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

