Influence of Different Levels of Lipoic Acid Synthase Gene Expression on Diabetic Nephropathy
- PMID: 27706190
- PMCID: PMC5051822
- DOI: 10.1371/journal.pone.0163208
Influence of Different Levels of Lipoic Acid Synthase Gene Expression on Diabetic Nephropathy
Abstract
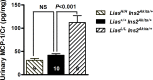
Oxidative stress is implicated in the pathogenesis of diabetic nephropathy (DN) but outcomes of many clinical trials are controversial. To define the role of antioxidants in kidney protection during the development of diabetic nephropathy, we have generated a novel genetic antioxidant mouse model with over- or under-expression of lipoic acid synthase gene (Lias). These models have been mated with Ins2Akita/+ mice, a type I diabetic mouse model. We compare the major pathologic changes and oxidative stress status in two new strains of the mice with controls. Our results show that Ins2Akita/+ mice with under-expressed Lias gene, exhibit higher oxidative stress and more severe DN features (albuminuria, glomerular basement membrane thickening and mesangial matrix expansion). In contrast, Ins2Akita/+ mice with highly-expressed Lias gene display lower oxidative stress and less DN pathologic changes. Our study demonstrates that strengthening endogenous antioxidant capacity could be an effective strategy for prevention and treatment of DN.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Overexpression of lipoic acid synthase gene alleviates diabetic nephropathy of Leprdb/db mice.BMJ Open Diabetes Res Care. 2021 Jun;9(1):e002260. doi: 10.1136/bmjdrc-2021-002260. BMJ Open Diabetes Res Care. 2021. PMID: 34183321 Free PMC article.
-
Reduced expression of lipoic acid synthase accelerates diabetic nephropathy.J Am Soc Nephrol. 2012 Jan;23(1):103-11. doi: 10.1681/ASN.2011010003. Epub 2011 Oct 21. J Am Soc Nephrol. 2012. PMID: 22021711 Free PMC article.
-
α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy.J Diabetes Complications. 2011 May-Jun;25(3):193-201. doi: 10.1016/j.jdiacomp.2010.07.004. Epub 2010 Aug 30. J Diabetes Complications. 2011. PMID: 20801062 Free PMC article.
-
A sensitized screen of N-ethyl-N-nitrosourea-mutagenized mice identifies dominant mutants predisposed to diabetic nephropathy.J Am Soc Nephrol. 2007 Jan;18(1):103-12. doi: 10.1681/ASN.2006020164. Epub 2006 Dec 6. J Am Soc Nephrol. 2007. PMID: 17151334
-
Diabetic kidney lesions of GIPRdn transgenic mice: podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis.Am J Physiol Renal Physiol. 2009 Apr;296(4):F819-29. doi: 10.1152/ajprenal.90665.2008. Epub 2009 Feb 11. Am J Physiol Renal Physiol. 2009. PMID: 19211686
Cited by
-
Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction.EMBO Rep. 2020 Aug 5;21(8):e49583. doi: 10.15252/embr.201949583. Epub 2020 Jul 9. EMBO Rep. 2020. PMID: 32648369 Free PMC article.
-
The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression.Front Oncol. 2023 Feb 28;13:1117164. doi: 10.3389/fonc.2023.1117164. eCollection 2023. Front Oncol. 2023. PMID: 36925927 Free PMC article. Review.
-
Overexpression of Lias Gene Alleviates Cadmium-Induced Kidney Injury in Mice Involving Multiple Effects: Metabolism, Oxidative Stress, and Inflammation.Biol Trace Elem Res. 2024 Jun;202(6):2797-2811. doi: 10.1007/s12011-023-03883-x. Epub 2023 Oct 7. Biol Trace Elem Res. 2024. PMID: 37804446
-
Overexpression of lipoic acid synthase gene alleviates diabetic nephropathy of Leprdb/db mice.BMJ Open Diabetes Res Care. 2021 Jun;9(1):e002260. doi: 10.1136/bmjdrc-2021-002260. BMJ Open Diabetes Res Care. 2021. PMID: 34183321 Free PMC article.
-
New insights into immunomodulation via overexpressing lipoic acid synthase as a therapeutic potential to reduce atherosclerosis.Vascul Pharmacol. 2020 Oct-Nov;133-134:106777. doi: 10.1016/j.vph.2020.106777. Epub 2020 Aug 1. Vascul Pharmacol. 2020. PMID: 32750408 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

