Cell Blebbing in Confined Microfluidic Environments
- PMID: 27706201
- PMCID: PMC5051935
- DOI: 10.1371/journal.pone.0163866
Cell Blebbing in Confined Microfluidic Environments
Abstract
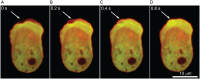
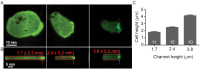
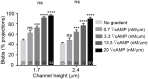
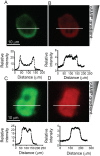
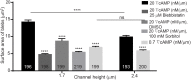
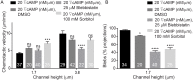
Migrating cells can extend their leading edge by forming myosin-driven blebs and F-actin-driven pseudopods. When coerced to migrate in resistive environments, Dictyostelium cells switch from using predominately pseudopods to blebs. Bleb formation has been shown to be chemotactic and can be influenced by the direction of the chemotactic gradient. In this study, we determine the blebbing responses of developed cells of Dictyostelium discoideum to cAMP gradients of varying steepness produced in microfluidic channels with different confining heights, ranging between 1.7 μm and 3.8 μm. We show that microfluidic confinement height, gradient steepness, buffer osmolarity and Myosin II activity are important factors in determining whether cells migrate with blebs or with pseudopods. Dictyostelium cells were observed migrating within the confines of microfluidic gradient channels. When the cAMP gradient steepness is increased from 0.7 nM/μm to 20 nM/μm, cells switch from moving with a mixture of blebs and pseudopods to moving only using blebs when chemotaxing in channels with confinement heights less than 2.4 μm. Furthermore, the size of the blebs increases with gradient steepness and correlates with increases in myosin-II localization at the cell cortex. Reduction of intracellular pressure by high osmolarity buffer or inhibition of myosin-II by blebbistatin leads to a decrease in bleb formation and bleb size. Together, our data reveal that the protrusion type formed by migrating cells can be influenced by the channel height and the steepness of the cAMP gradient, and suggests that a combination of confinement-induced myosin-II localization and cAMP-regulated cortical contraction leads to increased intracellular fluid pressure and bleb formation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Bleb-driven chemotaxis of Dictyostelium cells.J Cell Biol. 2014 Mar 17;204(6):1027-44. doi: 10.1083/jcb.201306147. Epub 2014 Mar 10. J Cell Biol. 2014. PMID: 24616222 Free PMC article.
-
How blebs and pseudopods cooperate during chemotaxis.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11703-8. doi: 10.1073/pnas.1322291111. Epub 2014 Jul 29. Proc Natl Acad Sci U S A. 2014. PMID: 25074921 Free PMC article.
-
Pressure sensing through Piezo channels controls whether cells migrate with blebs or pseudopods.Proc Natl Acad Sci U S A. 2020 Feb 4;117(5):2506-2512. doi: 10.1073/pnas.1905730117. Epub 2020 Jan 21. Proc Natl Acad Sci U S A. 2020. PMID: 31964823 Free PMC article.
-
Moving forward: mechanisms of chemoattractant gradient sensing.Physiology (Bethesda). 2004 Oct;19:300-8. doi: 10.1152/physiol.00017.2004. Physiology (Bethesda). 2004. PMID: 15381759 Review.
-
The role and regulation of blebs in cell migration.Curr Opin Cell Biol. 2013 Oct;25(5):582-90. doi: 10.1016/j.ceb.2013.05.005. Epub 2013 Jun 17. Curr Opin Cell Biol. 2013. PMID: 23786923 Free PMC article. Review.
Cited by
-
A flow-based microfluidic device for spatially quantifying intracellular calcium ion activity during cellular electrotaxis.Biomicrofluidics. 2019 Nov 7;13(6):064107. doi: 10.1063/1.5124846. eCollection 2019 Nov. Biomicrofluidics. 2019. PMID: 31737156 Free PMC article.
-
Modeling the dynamics of actin and myosin during bleb stabilization.bioRxiv [Preprint]. 2023 Oct 27:2023.10.26.564082. doi: 10.1101/2023.10.26.564082. bioRxiv. 2023. PMID: 37961169 Free PMC article. Preprint.
-
Three-Dimensional Printing Enabled Droplet Microfluidic Device for Real-Time Monitoring of Single-Cell Viability and Blebbing Activity.Micromachines (Basel). 2023 Jul 28;14(8):1521. doi: 10.3390/mi14081521. Micromachines (Basel). 2023. PMID: 37630057 Free PMC article.
-
Mechanochemical Signaling Directs Cell-Shape Change.Biophys J. 2017 Jan 24;112(2):207-214. doi: 10.1016/j.bpj.2016.12.015. Biophys J. 2017. PMID: 28122209 Free PMC article. Review.
-
Biophysical modeling identifies an optimal hybrid amoeboid-mesenchymal mechanism for maximal T cell migration speeds.bioRxiv [Preprint]. 2025 May 23:2023.10.29.564655. doi: 10.1101/2023.10.29.564655. bioRxiv. 2025. PMID: 39026744 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

