Fourth-Generation Progestins Inhibit 3β-Hydroxysteroid Dehydrogenase Type 2 and Modulate the Biosynthesis of Endogenous Steroids
- PMID: 27706226
- PMCID: PMC5051719
- DOI: 10.1371/journal.pone.0164170
Fourth-Generation Progestins Inhibit 3β-Hydroxysteroid Dehydrogenase Type 2 and Modulate the Biosynthesis of Endogenous Steroids
Abstract
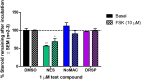
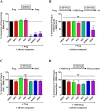
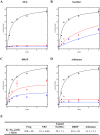
Progestins used in contraception and hormone replacement therapy are synthetic compounds designed to mimic the actions of the natural hormone progesterone and are classed into four consecutive generations. The biological actions of progestins are primarily determined by their interactions with steroid receptors, and factors such as metabolism, pharmacokinetics, bioavailability and the regulation of endogenous steroid hormone biosynthesis are often overlooked. Although some studies have investigated the effects of select progestins on a few steroidogenic enzymes, studies comparing the effects of progestins from different generations are lacking. This study therefore explored the putative modulatory effects of progestins on de novo steroid synthesis in the adrenal by comparing the effects of select progestins from the respective generations, on endogenous steroid hormone production by the H295R human adrenocortical carcinoma cell line. Ultra-performance liquid chromatography/tandem mass spectrometry analysis showed that the fourth-generation progestins, nestorone (NES), nomegestrol acetate (NoMAC) and drospirenone (DRSP), unlike the progestins selected from the first three generations, modulate the biosynthesis of several endogenous steroids. Subsequent assays performed in COS-1 cells expressing human 3βHSD2, suggest that these progestins modulate the biosynthesis of steroid hormones by inhibiting the activity of 3βHSD2. The Ki values determined for the inhibition of human 3βHSD2 by NES (9.5 ± 0.96 nM), NoMAC (29 ± 7.1 nM) and DRSP (232 ± 38 nM) were within the reported concentration ranges for the contraceptive use of these progestins in vivo. Taken together, our results suggest that newer, fourth-generation progestins may exert both positive and negative physiological effects via the modulation of endogenous steroid hormone biosynthesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
New progestagens for contraceptive use.Hum Reprod Update. 2006 Mar-Apr;12(2):169-78. doi: 10.1093/humupd/dmi046. Epub 2005 Nov 16. Hum Reprod Update. 2006. PMID: 16291771 Review.
-
An overview of nomegestrol acetate selective receptor binding and lack of estrogenic action on hormone-dependent cancer cells.J Steroid Biochem Mol Biol. 2003 Nov;87(2-3):111-22. doi: 10.1016/j.jsbmb.2003.08.003. J Steroid Biochem Mol Biol. 2003. PMID: 14672731 Review.
-
Effect of nomegestrol acetate on estrogen biosynthesis and transformation in MCF-7 and T47-D breast cancer cells.J Steroid Biochem Mol Biol. 2005 Jan;93(1):1-13. doi: 10.1016/j.jsbmb.2004.11.004. Epub 2005 Jan 26. J Steroid Biochem Mol Biol. 2005. PMID: 15748827 Review.
-
Differential metabolism of clinically-relevant progestogens in cell lines and tissue: Implications for biological mechanisms.J Steroid Biochem Mol Biol. 2019 May;189:145-153. doi: 10.1016/j.jsbmb.2019.02.010. Epub 2019 Feb 26. J Steroid Biochem Mol Biol. 2019. PMID: 30822501 Free PMC article.
-
Comparative analysis of the effects of nomegestrol acetate/17 β-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea.Eur J Contracept Reprod Health Care. 2015;20(4):296-307. doi: 10.3109/13625187.2015.1016154. Epub 2015 Feb 25. Eur J Contracept Reprod Health Care. 2015. PMID: 25712537 Clinical Trial.
Cited by
-
Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy.Biochem Biophys Res Commun. 2017 Sep 9;491(1):140-146. doi: 10.1016/j.bbrc.2017.07.063. Epub 2017 Jul 12. Biochem Biophys Res Commun. 2017. PMID: 28711501 Free PMC article.
-
Oral contraceptives containing ethinyl estradiol and drospirenone increase hydroxylation and methylation of endogenous estrogen but not genotoxic estrogen DNA-adduct formation.Sci Rep. 2025 Aug 26;15(1):31468. doi: 10.1038/s41598-025-16892-8. Sci Rep. 2025. PMID: 40858744 Free PMC article.
References
-
- Speroff L, A Clinical Guide for Contraception. 2nd ed 1996: Baltimore: Williams & Wilkins, Baltimore, MD.
-
- Stanczyk FZ. Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev Endocr Metab Disord. 2002; 3(3): 211–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

